A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-SOLUTION-All Questions
- Vapour pressure of pure A is 70 mm of Hg at 25^(@)C. If it forms an id...
Text Solution
|
- Lowering of vapour pressure is highest for
Text Solution
|
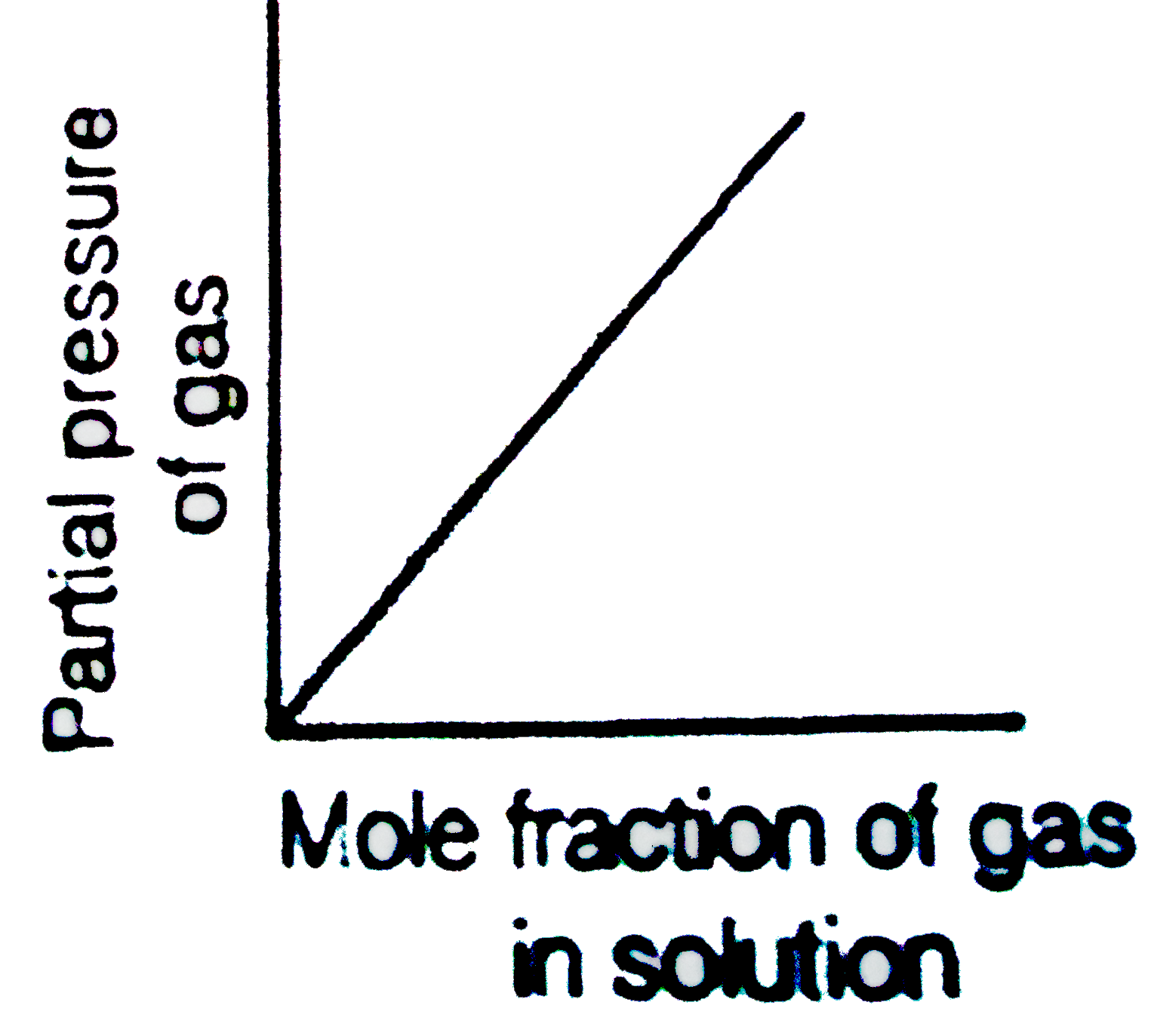
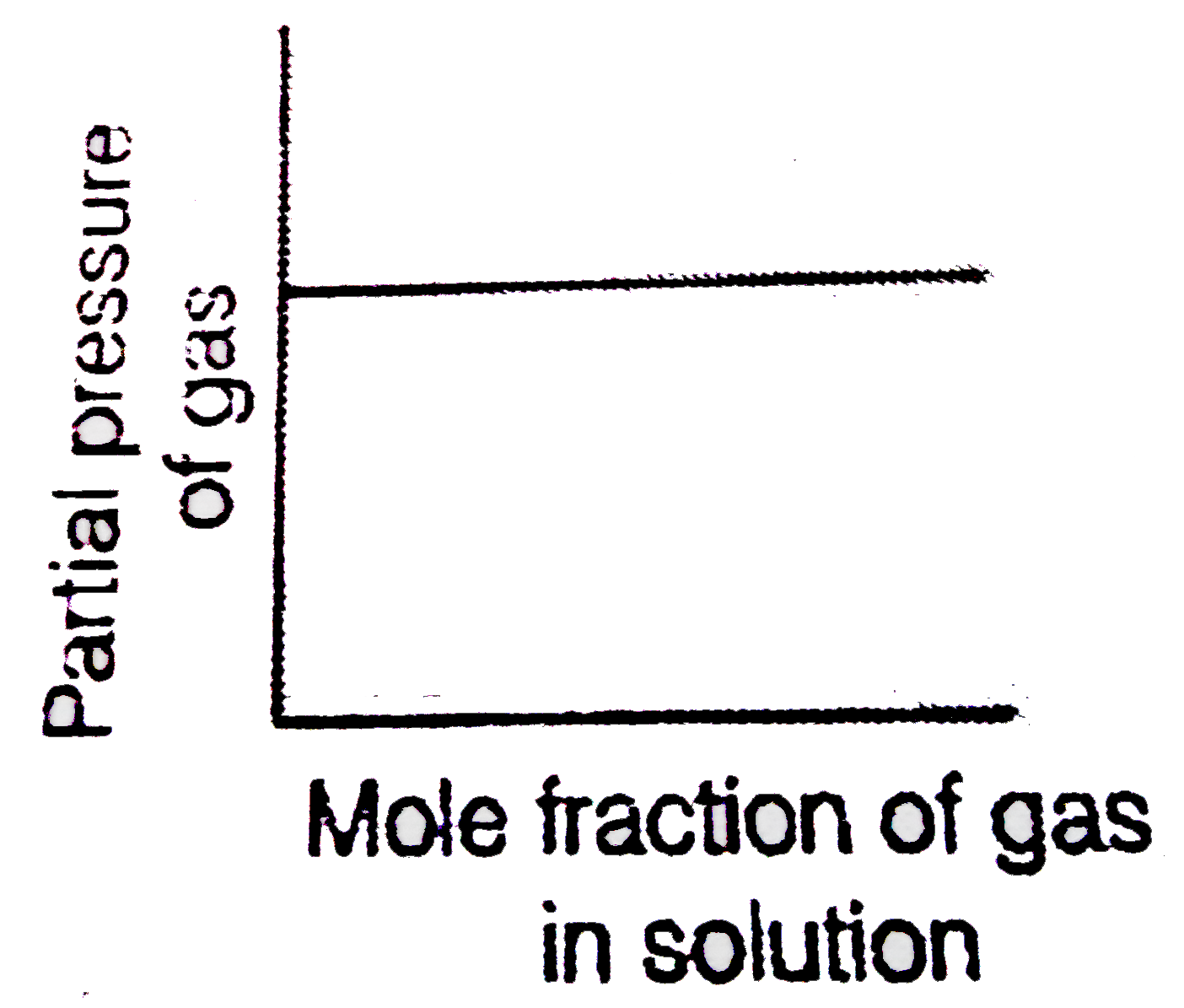
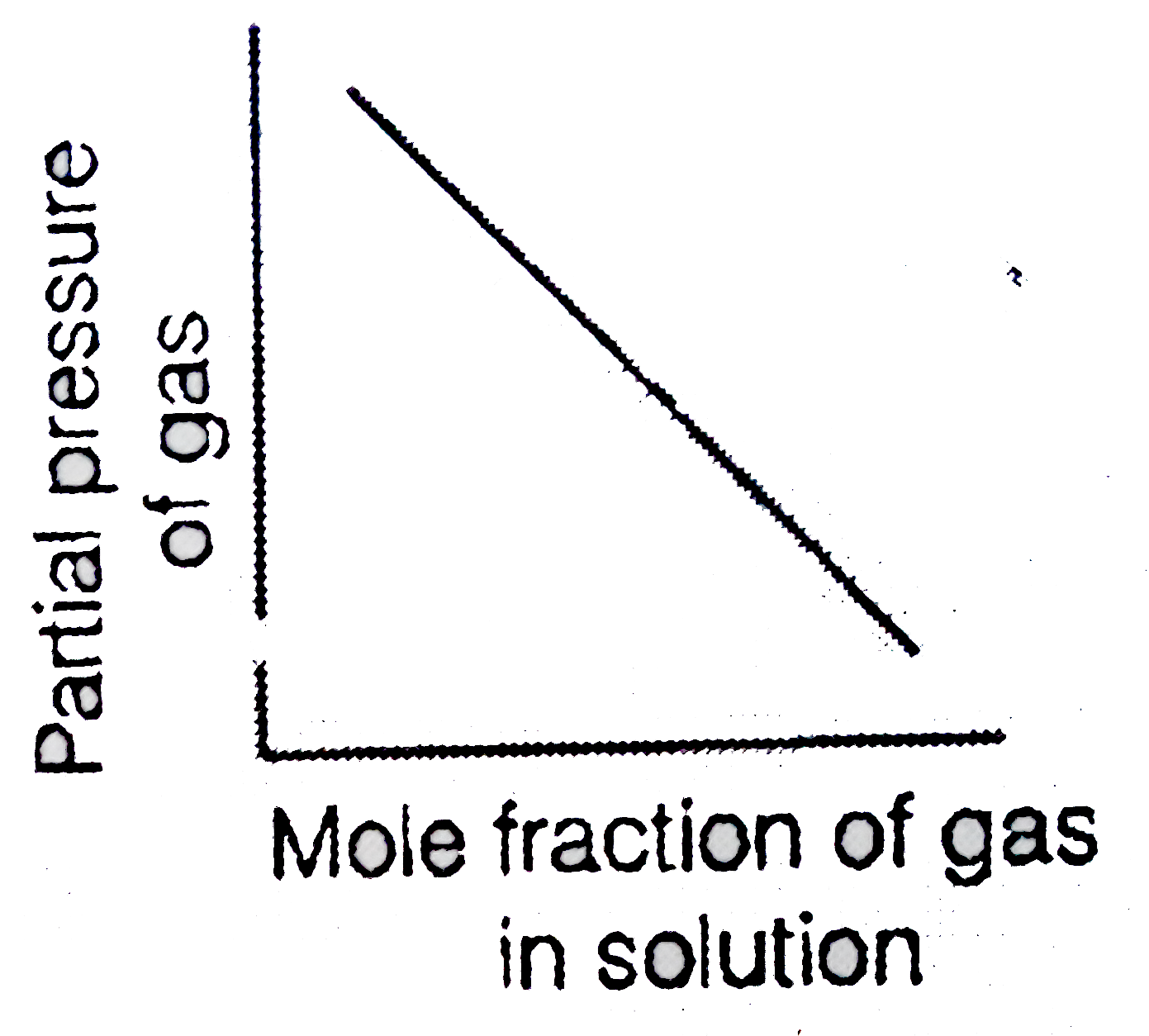
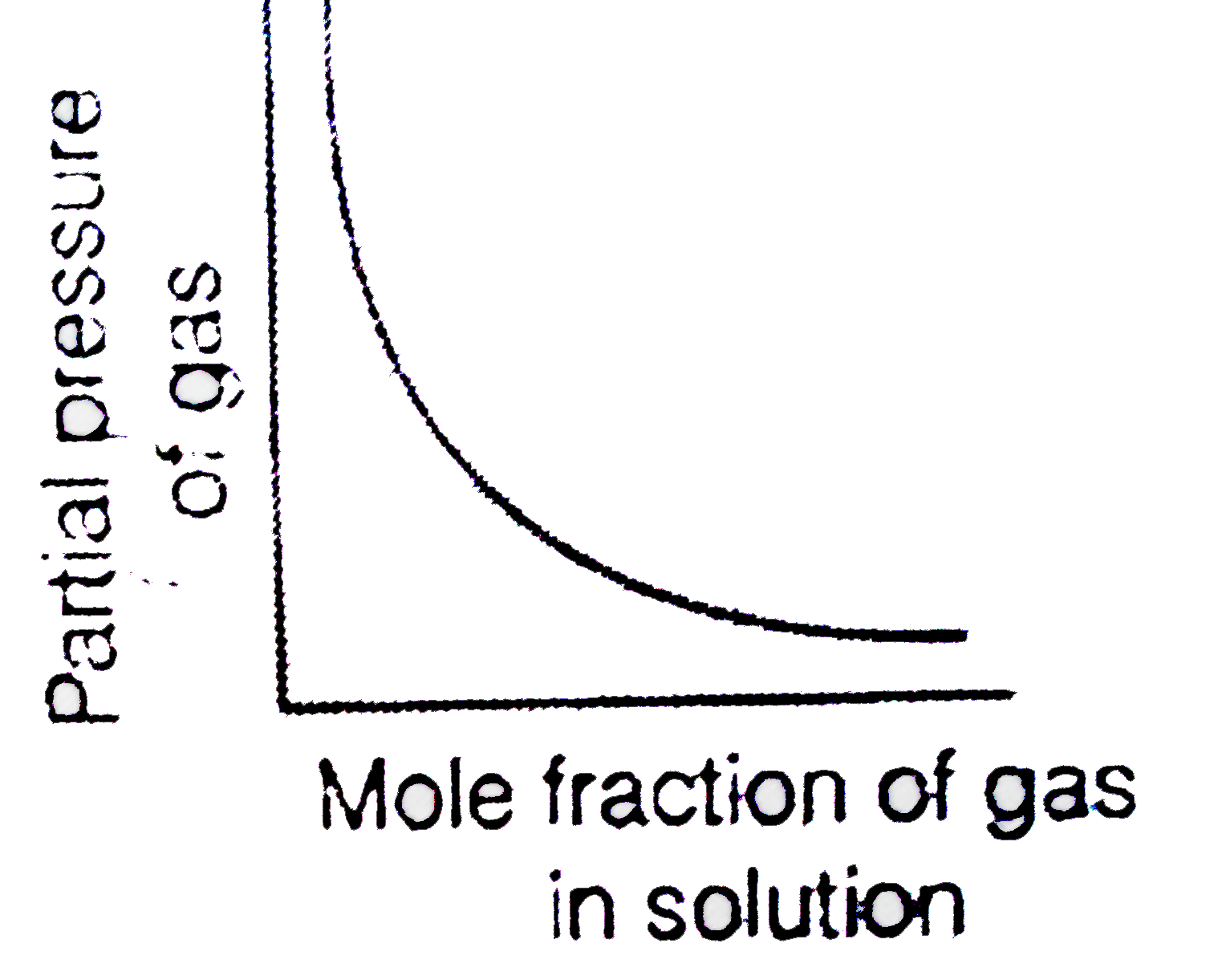
- Which of the following curves represent the Henry's law
Text Solution
|
- If p^(@) and p(a) are the vapour pressures of the solvent and solution...
Text Solution
|
- Which one of them is more volatile component?
Text Solution
|
- The elevation in boiling point would be highest for
Text Solution
|
- A 0.2 molal aqueous solution of weak acid (HX) is 20% ionised. The fre...
Text Solution
|
- The vant Hoff factor for a very dilute solution of Fe(2)(SO(4))(3) is
Text Solution
|
- The latent heat of vaporisation of water is 9700 "Cal/mole" and if the...
Text Solution
|
- The relative lowering of vapour pressure of an aqueous solution contai...
Text Solution
|
- The osmotic pressure of one molar solution at 27^(@)C is
Text Solution
|
- The freezing point of 0.1 M solution of glucose is -1.86^(@)C. If an e...
Text Solution
|
- The van't Hoff factor for 0.1 M Ba(NO(3))(2) solution is 2.74. The deg...
Text Solution
|
- In a 0.2 molal aqueous solution of a week acid HX, the degree of ionis...
Text Solution
|
- Acetic acid exists in benzene solution in the dimeric form. In an actu...
Text Solution
|
- A compound X undergoes tetramerisation in a given organic solvent. The...
Text Solution
|
- The freezing point (in .^@C) of solution containing 0.1 g of K(3)[Fe(C...
Text Solution
|
- 0.004 M Na(2)SO(4) is isotonic with 0.01 M glucose. Degree of dissocia...
Text Solution
|
- Colligative properties of a solution depends upon
Text Solution
|
- Among the following 0.10 m aqueous solutions, which one will exhibit t...
Text Solution
|