A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-JEE MOCK TEST 9-CHEMISTRY
- IUPAC name of is :
Text Solution
|
- For a given one mole of ideal gas kept at 6.5 atm in a container of ca...
Text Solution
|
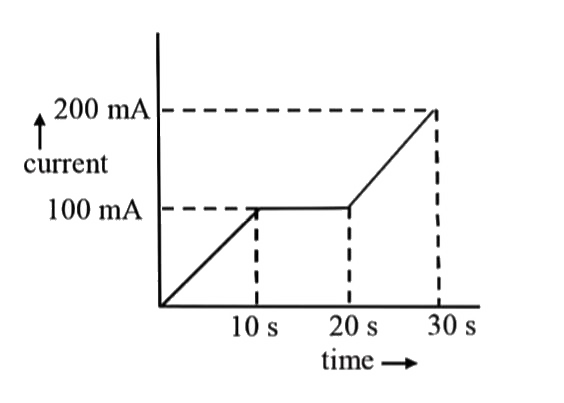
- In a Cu-voltameter, mass deposited in 30s is 'm' g. If the time -curre...
Text Solution
|
- Let the solubilities of Agbr in water and in 0.01M caBr(2), 0.01M KBr,...
Text Solution
|
- Which of the following pair of compounds is expected to exhibit same c...
Text Solution
|
- Among the following, the molecule with the highest dipole moment is :
Text Solution
|
- Determine the number of stereoisomers in the given compound
Text Solution
|
- Which fo the following processes involves smelting
Text Solution
|
- Which of the following is not formed in iodoform reaction ?
Text Solution
|
- The compound A on heating gives a colourless gas and a residue that is...
Text Solution
|
- TlI(3) is an ionic compound. In the aqueous solution it provides -
Text Solution
|
Text Solution
|
- What is correct increasing oreder of bond lengths of bond indicated as...
Text Solution
|
- Identify the correct decreasing order of acid strength for the followi...
Text Solution
|
- The equilibrium constant K(p(1)) and K(p(2)) for the reactions XhArr2Y...
Text Solution
|
- The correct statement on the isomerism associated with the follow...
Text Solution
|
- The de-Broglie wavelength of an alpha-particles at a voltage V is ...
Text Solution
|
- An amino acid having isoelectric point below 7 ( at 25^(@)C ) , when k...
Text Solution
|
- Chemisorption
Text Solution
|
- A 5.25% solution of a substance is isotonic with a 1.5% solution of ur...
Text Solution
|