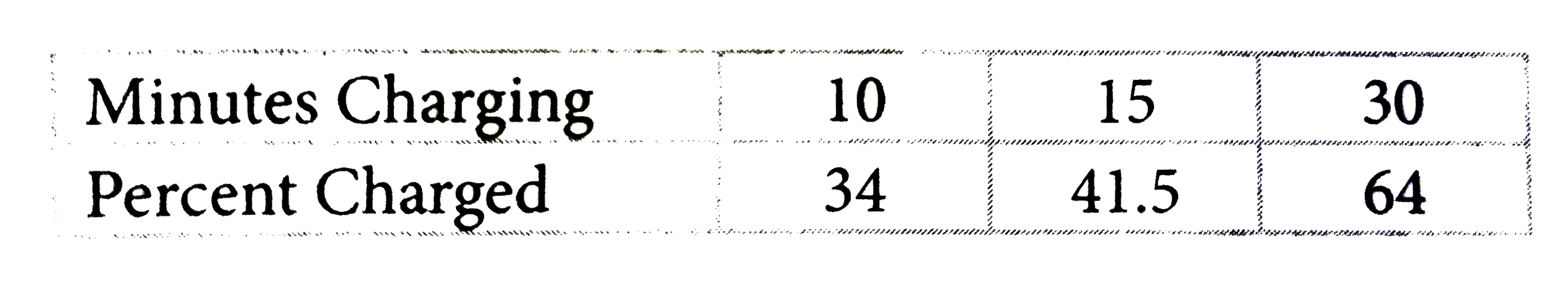A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LINEAR EQUATIONS AND GRAPHS
KAPLAN|Exercise SOLVING EQUATIONS|1 VideosLINEAR EQUATIONS AND GRAPHS
KAPLAN|Exercise WORD PROBLEMS|1 VideosLINEAR EQUATIONS AND GRAPHS
KAPLAN|Exercise LINEAR GRAPH|1 VideosINEQUALITIES
KAPLAN|Exercise MODELING REAL-LIFE SITUATIONS WITH INEQUALITIES|1 VideosPAIRED PASSAGES AND PRIMARY SOURCE PASSAGES
KAPLAN|Exercise HOW MUCH HAVE YOU LEARNED|11 Videos
Similar Questions
Explore conceptually related problems
KAPLAN-LINEAR EQUATIONS AND GRAPHS-TRY YOUR OWN
- The final value, v, in a four-digit lock code is determined by multipl...
Text Solution
|
- A pizzeria's top-selling pizzas are The Works and The Hawaiian. The Wo...
Text Solution
|
- A student opens a checking account when she starts a new job so that h...
Text Solution
|
- Milk starts a job at which his starting salary is $25500 per year. He ...
Text Solution
|
- Line A passes through the cooredinate points (-2/5, 0) and (0,1). Whic...
Text Solution
|
- In the xy-plane, the point (4,7) lies on the line t, which is perpendi...
Text Solution
|
- The graph shows the correlation between ambient air temperature, t, in...
Text Solution
|
- A freight airline charges a flat fee to airmil a box, plus an addition...
Text Solution
|
- Xia is charging her laptop. She records the battery charge for the fir...
Text Solution
|
- Remember that the SAT doesn't ask you to show your work. If you find t...
Text Solution
|
- Which value of x makes the equation 8/5(x+(33)/(12))=16 true ?
Text Solution
|
- The graph above shows the cost of joining and buying music from a musi...
Text Solution
|
- If 3/4 y =6-1/3c, then what is the value of 2x + 9/2y ?
Text Solution
|
- Three years ago, Madison High School started charging an admission fee...
Text Solution
|
- Which of the following equations best, describes the linear relationsh...
Text Solution
|
- Brain and Jared live in the same apartment comlex and both bike to and...
Text Solution
|
- When graphing a linear equation that is written in the form y = mx+b, ...
Text Solution
|
- The graph of a line in the xy-plane passes through the points (5,4) an...
Text Solution
|
- Line f in th exy-plane passes through the origin and has a slope of -2...
Text Solution
|
- V =1/3 pi ((d)/(2)) ^(2) h A circle of rubber with a constant diamet...
Text Solution
|