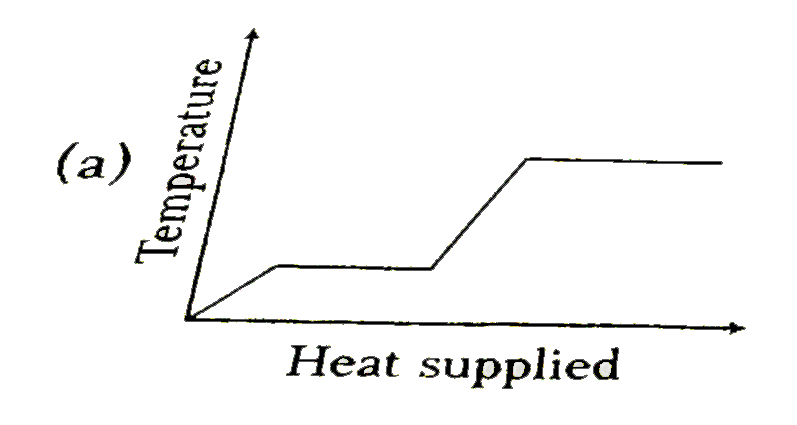
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CALORIMETRY AND HEAT TRANSFER
DC PANDEY|Exercise Assertion and reason|17 VideosCALORIMETRY AND HEAT TRANSFER
DC PANDEY|Exercise Match the columns|4 VideosCALORIMETRY AND HEAT TRANSFER
DC PANDEY|Exercise Check points 16.4|25 VideosCALORIMETRY & HEAT TRANSFER
DC PANDEY|Exercise Level 2 Subjective|14 VideosCENTRE OF MASS
DC PANDEY|Exercise Medical entrances gallery|27 Videos
Similar Questions
Explore conceptually related problems
DC PANDEY-CALORIMETRY AND HEAT TRANSFER-Taking it together
- A wall has two layers A and B each made of different materials. The la...
Text Solution
|
- A body cools from 50^(@)C to 40^(@)C in 5 min. The surroundings temper...
Text Solution
|
- A block of ice at -10^@C is slowly heated and converted to steam at 10...
Text Solution
|
- If one kilogram water at 100^(@)C is vapourised in open atmosphere. Th...
Text Solution
|
- A liquid cools from 50^(@)C to 45^(@)C in 5 minutes and from 45^(@)C ...
Text Solution
|
- The spectrum of a black body at two temperatures 27^(@)C and 327^(@)C ...
Text Solution
|
- The graph, shown in the adjacent diagram, represents the variation of ...
Text Solution
|
- Two substances A and B of equal mass m are heated by uniform rate of 6...
Text Solution
|
- Sunrays are allowed to fall on a lens of diameter 20 cm. They are then...
Text Solution
|
- If a body coated black at 600K surrounded by atmosphere at 300 K has c...
Text Solution
|
- The power radiated by a black body is P, and it radiates maximum energ...
Text Solution
|
- In the figure ABC is a conducting rod whose lateral surfaces are insul...
Text Solution
|
- Two similar rods are joined as shown in figure. Then temperature of ju...
Text Solution
|
- Five rods of same dimensions are arranged as shown in the figure. They...
Text Solution
|
- Three identical metal rods A, B and C are placed end to end and a temp...
Text Solution
|
- 0.3 Kg of hot coffee, which is at 70^(@)C, is poured into a cup of mas...
Text Solution
|
- A calorimeter contains 10 g of water at 20^(@)C. The temperature falls...
Text Solution
|
- 19 g of water at 30^@C and 5 g of ice at -20^@C are mixed together in ...
Text Solution
|
- Work done in converting 1 g of ice at -10^@C into steam at 100^@C is
Text Solution
|
- Two rigid boxes containing different ideal gases are placed on a table...
Text Solution
|