A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-JEE MOCK TEST 12-CHEMISTRY - SUBJECTIVE NUMERICAL
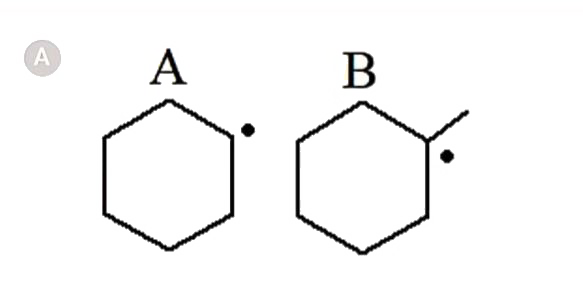
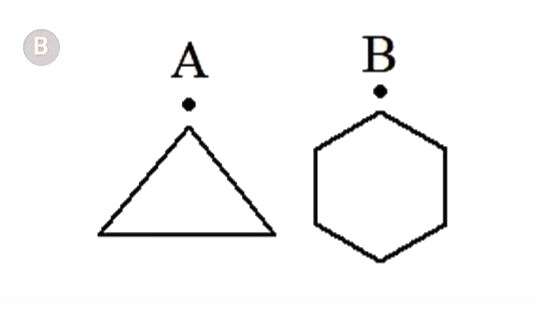
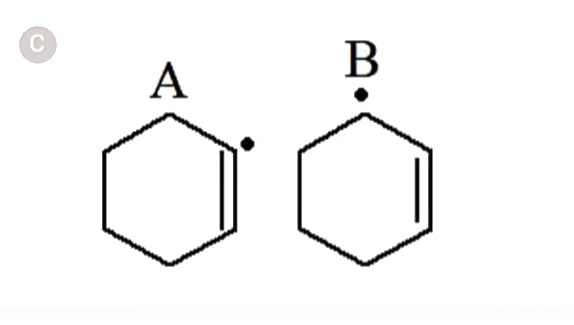
- In which of the following pairs A is more stable than B?
Text Solution
|
- For the electrochemical cell, Mg(s)|Mg^(2+) (aq. 1M)||Cu^(2+) (aq. 1...
Text Solution
|
- Among the compounds , Benzene, Carbon tetrachloride, Naphthalene, Benz...
Text Solution
|
- Number of incorrect statement are- (A) The pi bond between metal and...
Text Solution
|
- How many of the following pollutants are considered as non-viable part...
Text Solution
|
- Total number of electrophiles present in the following are NO(2)^(+)...
Text Solution
|