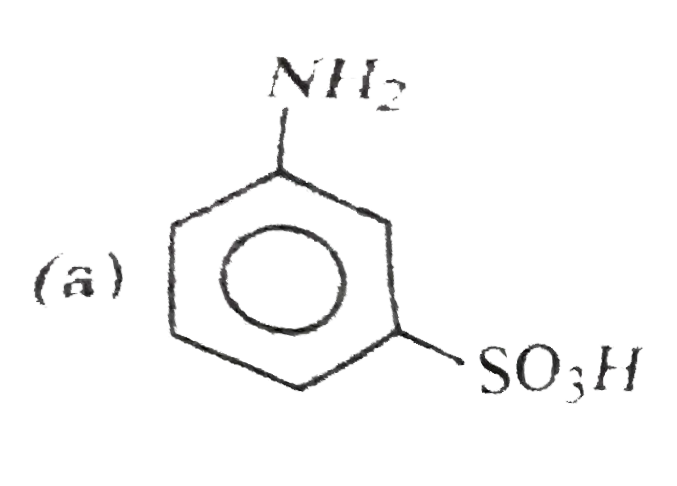
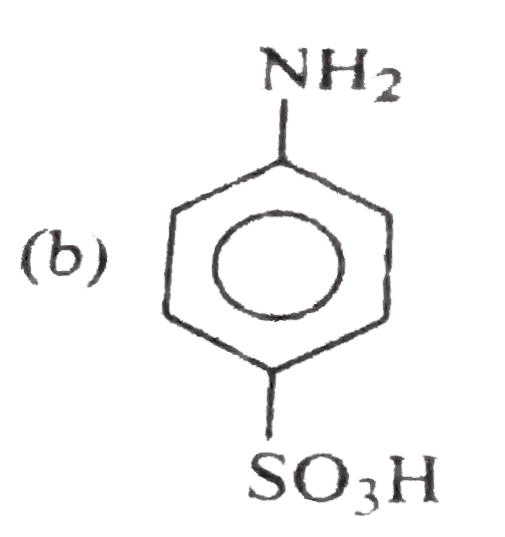
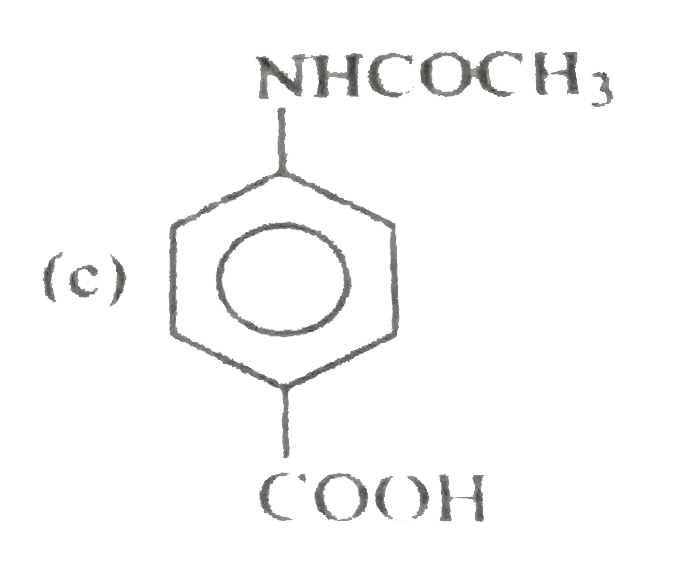
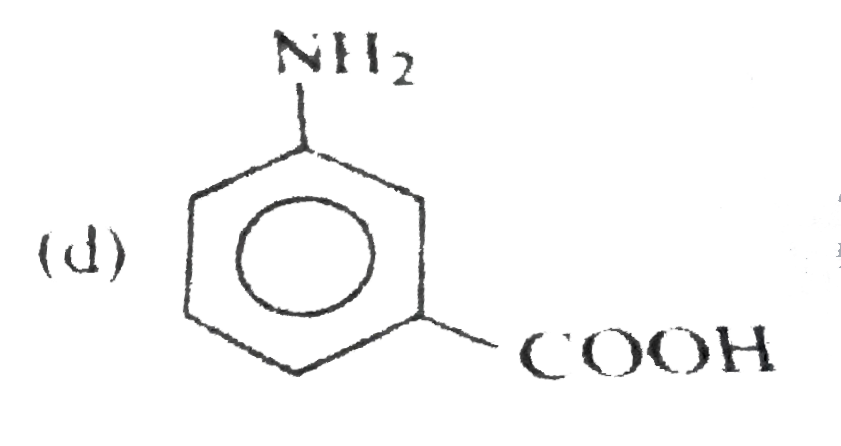
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A white crystalline solid 'X' give following chemical test : (i) i...
Text Solution
|
- A white crystalline compound (X) swells open heating and gives violet-...
Text Solution
|
- Statement I: Aniline on reaction with NaNO2HCl at 0^@C followed by cou...
Text Solution
|
- A white crystalline solid 'X' give following chemical test : (i) i...
Text Solution
|
- A: An organic compound on diazotisation followed by reaction with alka...
Text Solution
|
- A metal ion 'X' reacts with NaOH to give a white ppt. precipitate can ...
Text Solution
|
- Acetaldehyde form a white crystalline precipitate mixing with a soluti...
Text Solution
|
- A compound A has a molecules formula C(7)H(7)NO . On tratement with Br...
Text Solution
|
- Acetaldehyde form a white crystalline precipitate mixing with a soluti...
Text Solution
|