A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Level-VI
- A piece of zinc at a temperature of 20.0^(@) C weighing 63.38 g is ...
Text Solution
|
- Determine which of the following reaction at constant pressure represe...
Text Solution
|
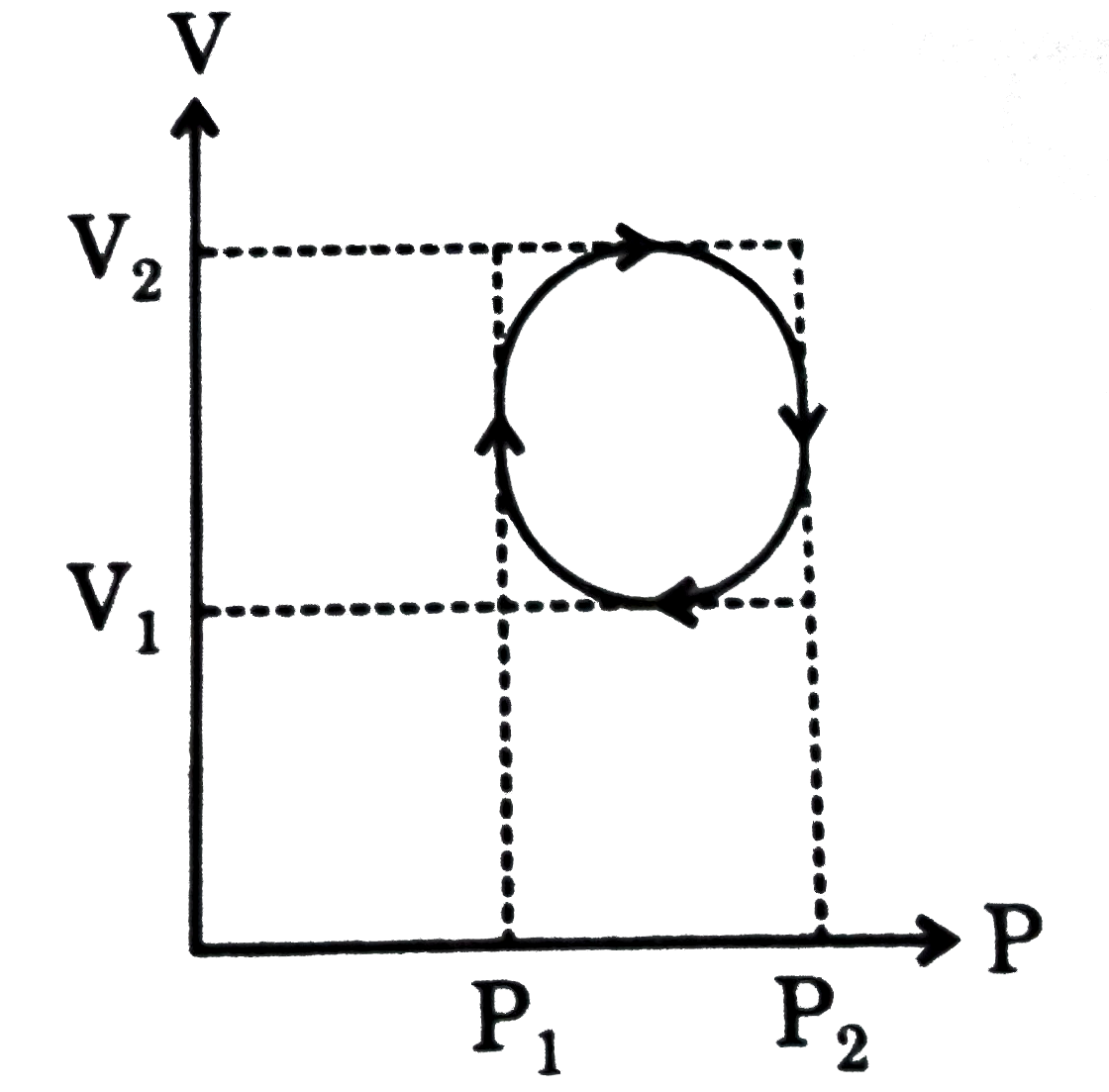
- In the cyclic process shown in P-V diagram the magnitude of work done ...
Text Solution
|
- One mole of a non-ideal gas undergoes a change of state (2.0atm,3.0L,9...
Text Solution
|
- The reaction CH(4) (g) + Cl(2) (g) rarr CH(3)Cl (g) + HCl (g) has Delt...
Text Solution
|
- The standard molar enthalpies of formation of cyclohexane (l) and benz...
Text Solution
|
- The value of log(10)K for a reaction A hArr B is (Given: Delta(f)H(298...
Text Solution
|
- Find DeltaG^(@)andDeltaH^(@) for the reaction CO(g)+(1)/(2)O(2)(g) t...
Text Solution
|
- In conversion of lime-stone to lime, CaCO(3(s)) to CaO((s)) + CO(2(g...
Text Solution
|
- The change in free energy accompainied by the isothermal reversible ex...
Text Solution
|
- The following curve represnets the variation of Gibbs function 'G' wit...
Text Solution
|
- Which correctly represents the entropy (s) of an isolated system duri...
Text Solution
|
- One mole of an ideal monoatomic gas expands isothermally against const...
Text Solution
|
- At 1000 K water vapour at 1 atm has been found to be dissociated into ...
Text Solution
|
- Which of the following graphs best illustrates the variation of entrop...
Text Solution
|
- During winters, moisture condenses in the form of dew and can be seen ...
Text Solution
|
- A reactions is spontaneous if
Text Solution
|
- Which of the following expressions are correct for an ideal gas ?
Text Solution
|
- Which of the following expression are incorrect for a perfect gas ?
Text Solution
|
- For an ideal gas, an illustration of three different paths A(B+C) and ...
Text Solution
|