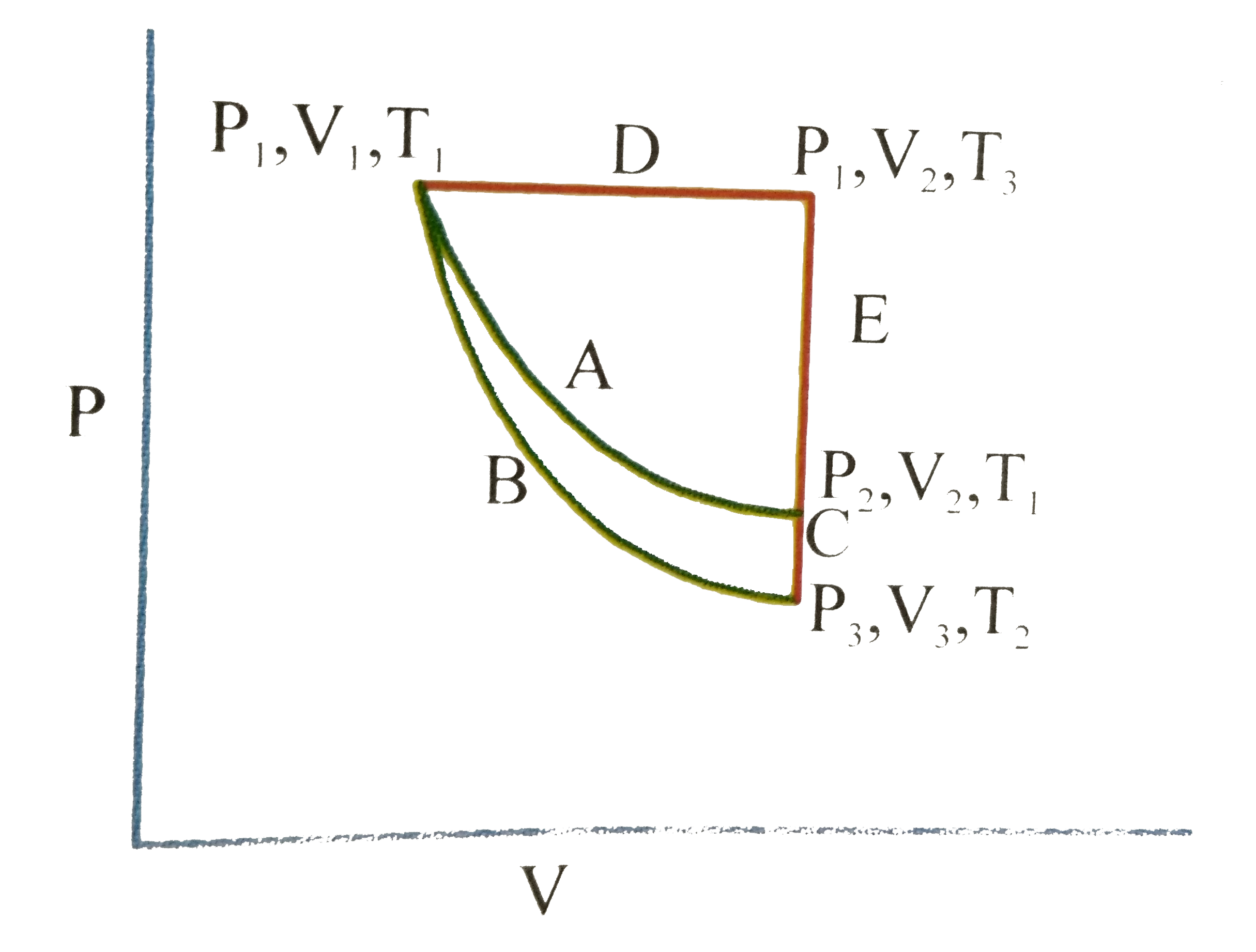
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Level-VI
- For an ideal gas, an illustration of three different paths A(B+C) and ...
Text Solution
|
- For an ideal gas, an illustratio of three different paths A(B+C) and (...
Text Solution
|
- For an ideal gas, an illustration of the different paths, A (B + C) an...
Text Solution
|
- Standard Gibb's energy of reaction (Delta(r )G^(@)) at a certain temp...
Text Solution
|
- Standard Gibb's energy of reaction (Delta(r )G^(@)) at a certain temp...
Text Solution
|
- Standard Gibb's energy of reaction (Delta(r )G^(@)) at a certain temp...
Text Solution
|
- Standard Gibb's energy of reaction (Delta(r )G^(@)) at a certain temp...
Text Solution
|
- Standard Gibb's energy of reaction (Delta(r )G^(@)) at a certain temp...
Text Solution
|
- 9.0 gm ice 0^(@)C is mixed with 36 gm of water at 50^(@)C in a thermal...
Text Solution
|
- 9.0 gm ice 0^(@)C is mixed with 36 gm of water at 50^(@)C in a thermal...
Text Solution
|
- 9.0 gm ice 0^(@)C is mixed with 36 gm of water at 50^(@)C in a thermal...
Text Solution
|
- 9.0 gm ice 0^(@)C is mixed with 36 gm of water at 50^(@)C in a thermal...
Text Solution
|
- Liquid water freezes at 273 K under external pressure of 1 atm. The pr...
Text Solution
|
- Liquid water freezes at 273 K under external pressure of 1 atm. The pr...
Text Solution
|
- The relation between change in internal energy(DeltaE)change in enthal...
Text Solution
|
- The relation between change in internal energy(DeltaE)change in enthal...
Text Solution
|
- The relation between change in internal energy(DeltaE)change in enthal...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|