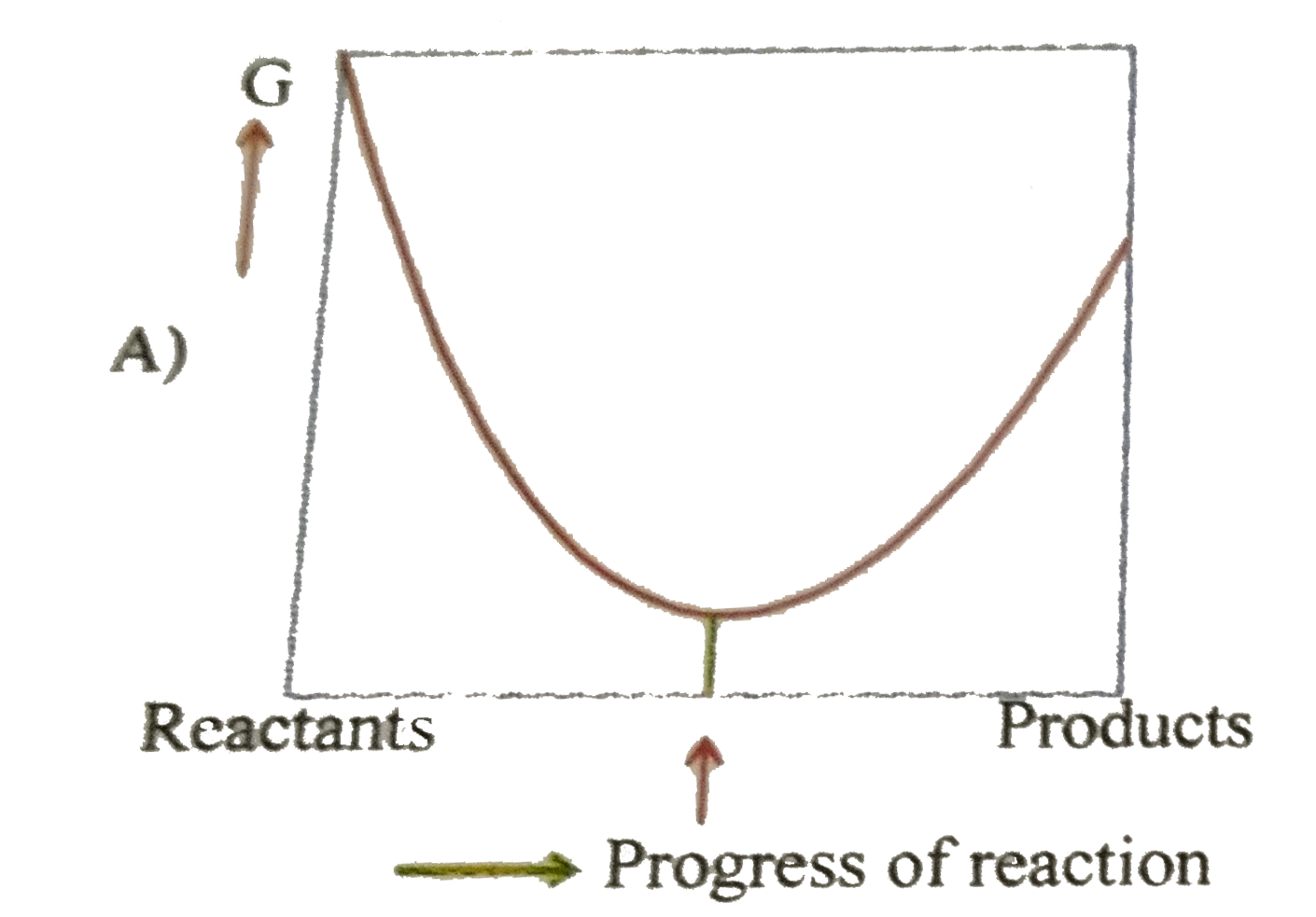
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Level-VI
- The relation between change in internal energy(DeltaE)change in enthal...
Text Solution
|
- The relation between change in internal energy(DeltaE)change in enthal...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- Match column - I with Colimn - II
Text Solution
|
- Match column - I with Colimn - II
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
Text Solution
|
- Match the following columns
Text Solution
|
Text Solution
|
- Staement -1: The heat absorbed during the isothermal expansion of an i...
Text Solution
|
- Work done during isothermal reversible process is given
Text Solution
|
- Statement-1: Heat of neutralization of HCl by NaOH is more than that b...
Text Solution
|
- Statement: The change in internal energy and change in heat enthalpy d...
Text Solution
|
- Statement -1: Entropy change in reversible adiabatic expansion of an i...
Text Solution
|
- The enthalpy change involved in the oxidation of glucose is -2880 kJ m...
Text Solution
|
- Calculate the enthalpy change when infinitely dilute solutions of CaCl...
Text Solution
|