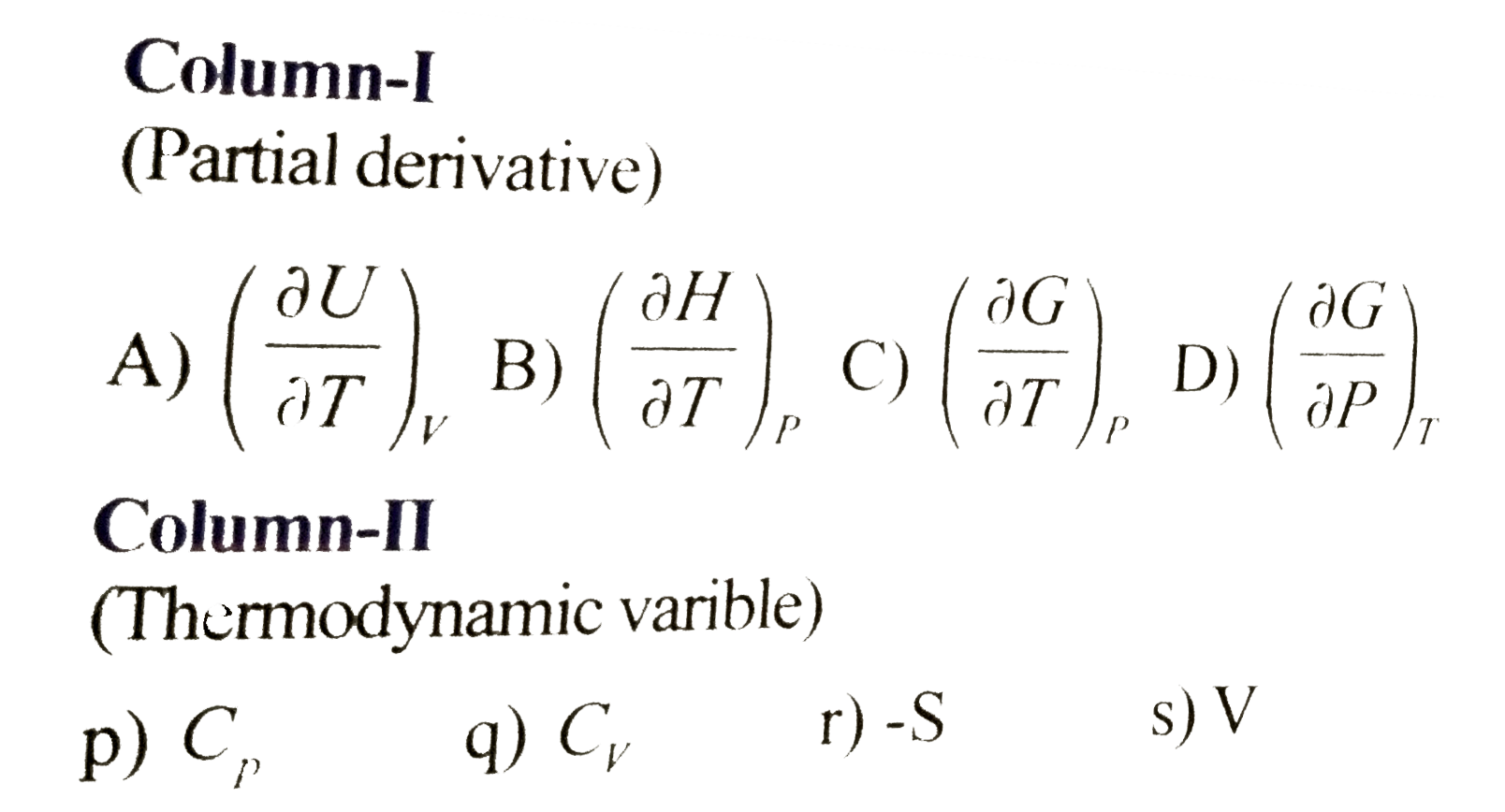Text Solution
Verified by Experts
The correct Answer is:
NARAYNA-THERMODYNAMICS-Level-VI
Text Solution
|
- Match the following columns
Text Solution
|
Text Solution
|
- Staement -1: The heat absorbed during the isothermal expansion of an i...
Text Solution
|
- Work done during isothermal reversible process is given
Text Solution
|
- Statement-1: Heat of neutralization of HCl by NaOH is more than that b...
Text Solution
|
- Statement: The change in internal energy and change in heat enthalpy d...
Text Solution
|
- Statement -1: Entropy change in reversible adiabatic expansion of an i...
Text Solution
|
- The enthalpy change involved in the oxidation of glucose is -2880 kJ m...
Text Solution
|
- Calculate the enthalpy change when infinitely dilute solutions of CaCl...
Text Solution
|
- An intimate mixture of ferric oxide and aluminium is used as solid fue...
Text Solution
|
- Calculate the enthalpy change for the following reversible process: ...
Text Solution
|
- Heat of neutralization between HCl and NaOH is -13.7 k.cal. If heat of...
Text Solution
|
- A gas occupies 2 litre at STP. It is provided 58.63 joule heat so that...
Text Solution
|
- A sample of ideal gas (gamma = 1.4) is heated at constant pressure. If...
Text Solution
|
- The free energy change when 1 mole of NaCl is dissolved in water at 29...
Text Solution
|
- Calculate the maximum work done in expanding 16g of oxygen at 300K occ...
Text Solution
|
- What is the entropy change for the conversion of one gram of ice to wa...
Text Solution
|
- The enthalpy of transition of crustalline boron to amorphous boron at ...
Text Solution
|
- At 0^(2)C, if enthalpy of fusion of ice is 1365 kcal/mol. The molar en...
Text Solution
|