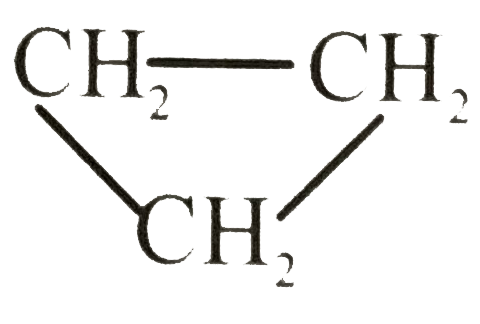
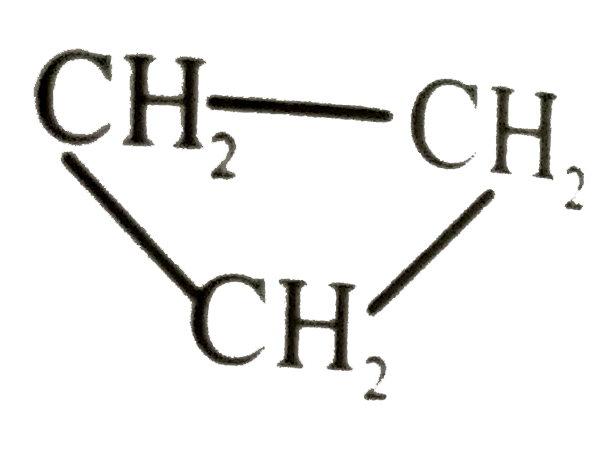
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Level-VI
- The standard ethelpy of combustion at 25^(@)C of hydrogen, cyclohexene...
Text Solution
|
- Using the data (all values are in kilocalorie per mole at 25^(@)C) giv...
Text Solution
|
- A gas mixture of 3.67L of ethylene and methane on complete combustion ...
Text Solution
|
- Determine the enthalpy change of the reaction. C(3)H(5) (g) + H(2) (g)...
Text Solution
|
- In order to get maximum calorific output a burner should have an optim...
Text Solution
|
- The polymerisation of ethylene to linear polyethylene is represented b...
Text Solution
|
- The standard molar enthalpies of formation of cyclohexane (l) and benz...
Text Solution
|
- The enthalpy change involved in the oxidation of glucose is -2880kJ mo...
Text Solution
|
- Compute the heat of formation of liquie methyl alcohol is kilojoule pe...
Text Solution
|
- Anhydrous AlCl(3) is covalent. From the date given below, predict whet...
Text Solution
|
- From the following data, calculate the enthalpy change for the combust...
Text Solution
|
- The standard heat of formation values of SF(6)(g), S(g), and F(g) are ...
Text Solution
|
- A sample of argon gas at 1atm pressure and 27^(@)C expands reversibly ...
Text Solution
|
- Show that the reaction CO(g) +(1//2)O(2)(g) rarr CO(2)(g) at 300K ...
Text Solution
|
- Diborane is a potential rocket fuel which undergoes combustion accordi...
Text Solution
|
- When 1pentyne (A) is treated with 4N alcoholic KOH at 175^(@)C, it is ...
Text Solution
|
- For the reaction 2CO +O(2) rarr 2CO(2), DeltaH =- 560 kJ, 2mol of ...
Text Solution
|
- C(v) values of He is always (3R)/(2) but C(v) values of H(2) is (3R)/(...
Text Solution
|
- An insulated vessel contains 1mole of a liquid, molar volume 100mL at ...
Text Solution
|
- In the reaction equilibrium N(2)O(4) hArr 2NO(2)(g) When 5 mol of ...
Text Solution
|
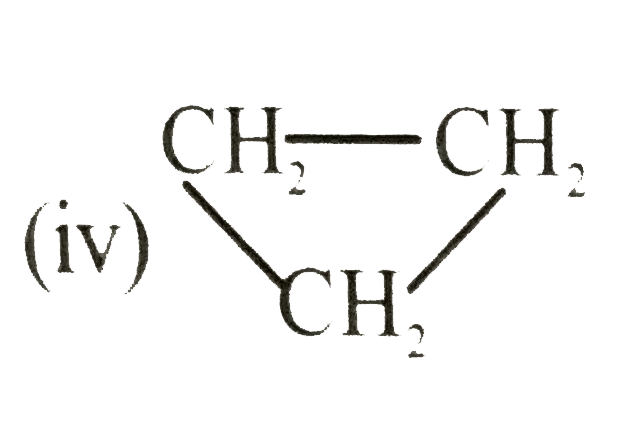 `(g) rarr C_(3) H_(6) (g) , Delta H = -33.0 kJ`
`(g) rarr C_(3) H_(6) (g) , Delta H = -33.0 kJ`