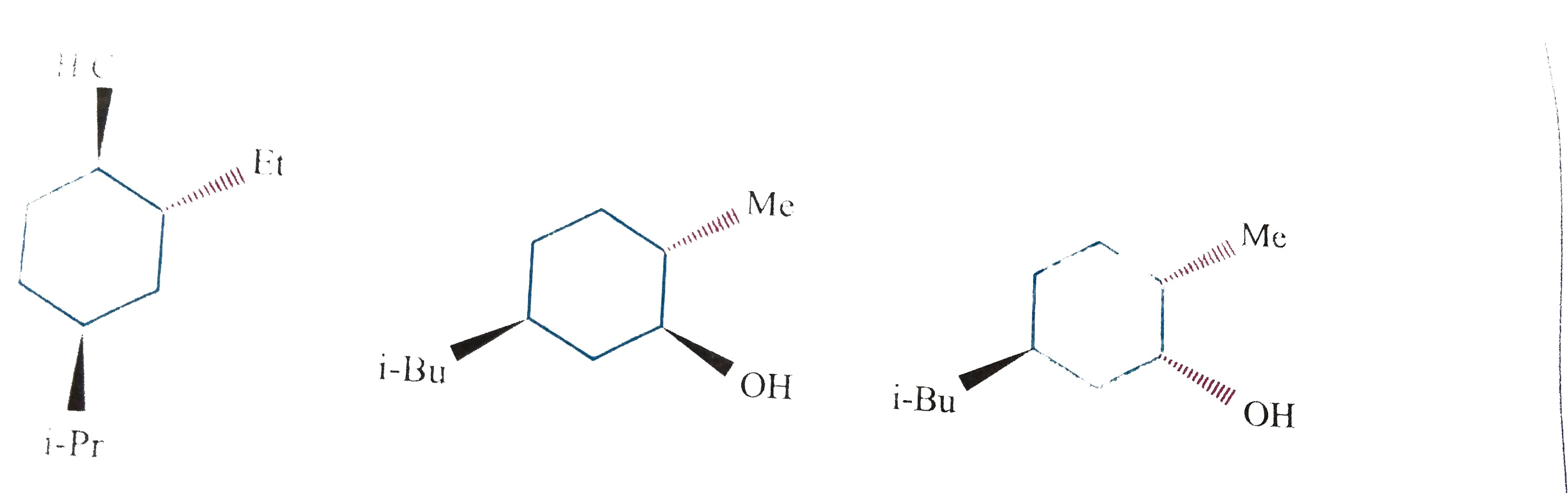
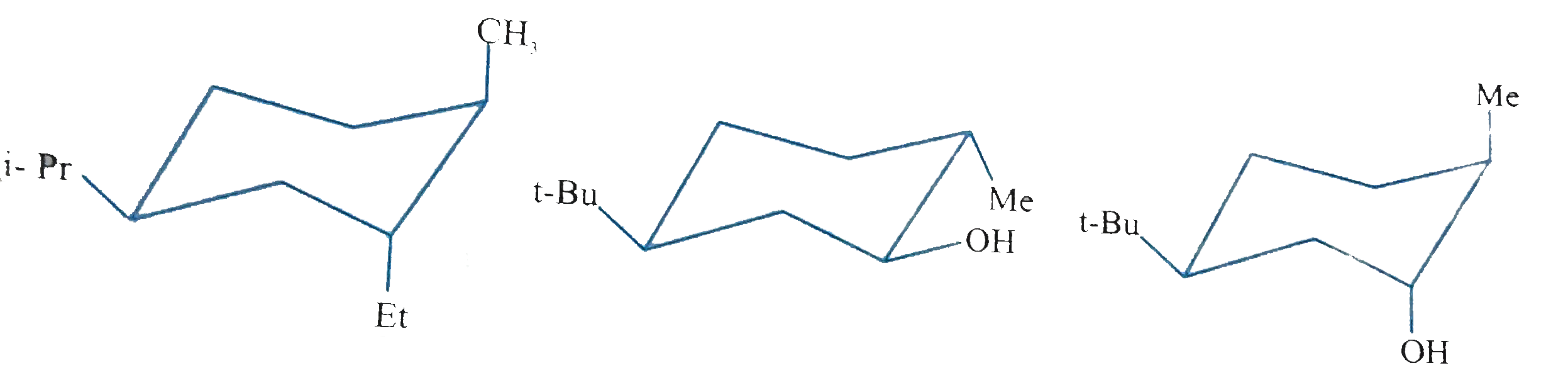
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-GENERAL ORGANIC CHEMISTRY-Matrix-Match type questions
- Draw the most stable configuration of
Text Solution
|
- Match the following Column I with Column II
Text Solution
|
- Macth the column I and II
Text Solution
|
- Match the following Column I with Column II
Text Solution
|
- Match the following
Text Solution
|
- Match the following compounds in colummn I with number of isomers in C...
Text Solution
|
- Match the following compounds in colummn I with configuration in Colum...
Text Solution
|
- Match the following
Text Solution
|