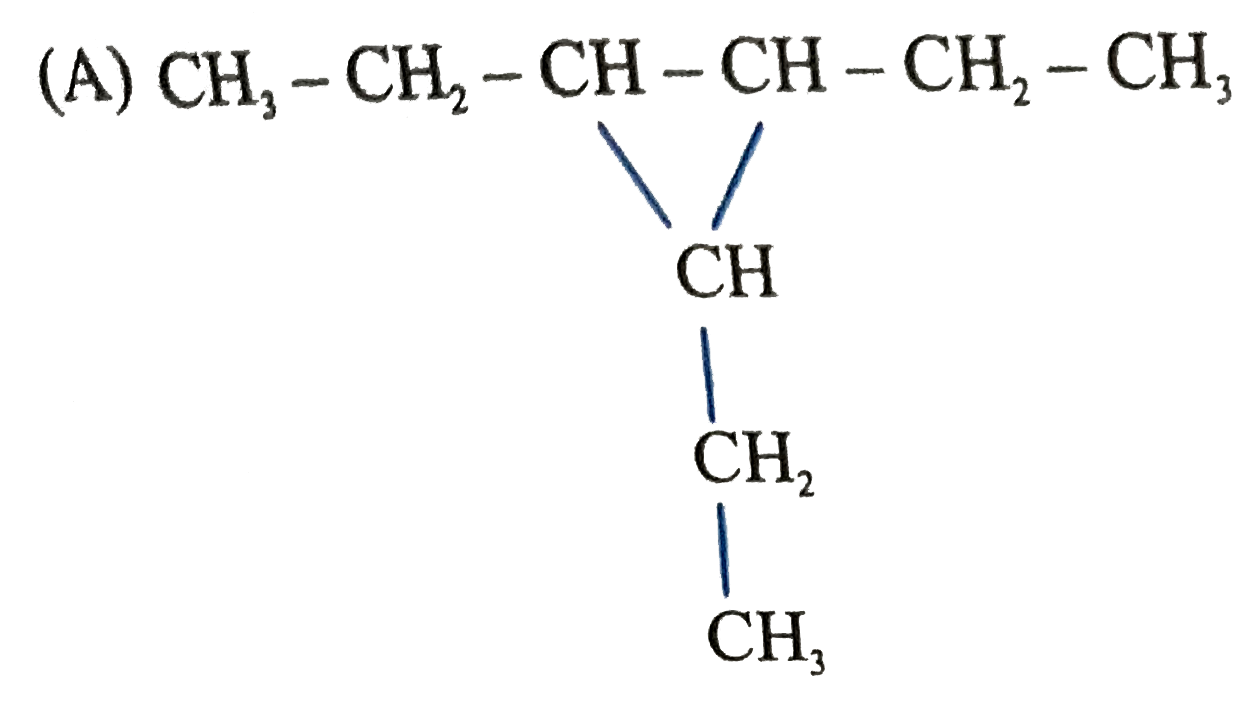
A
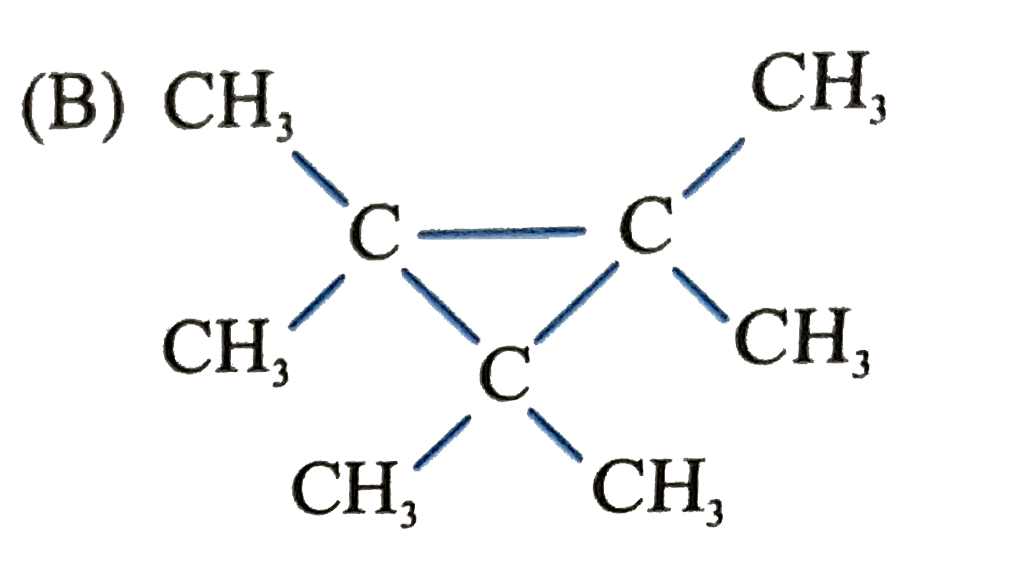
B
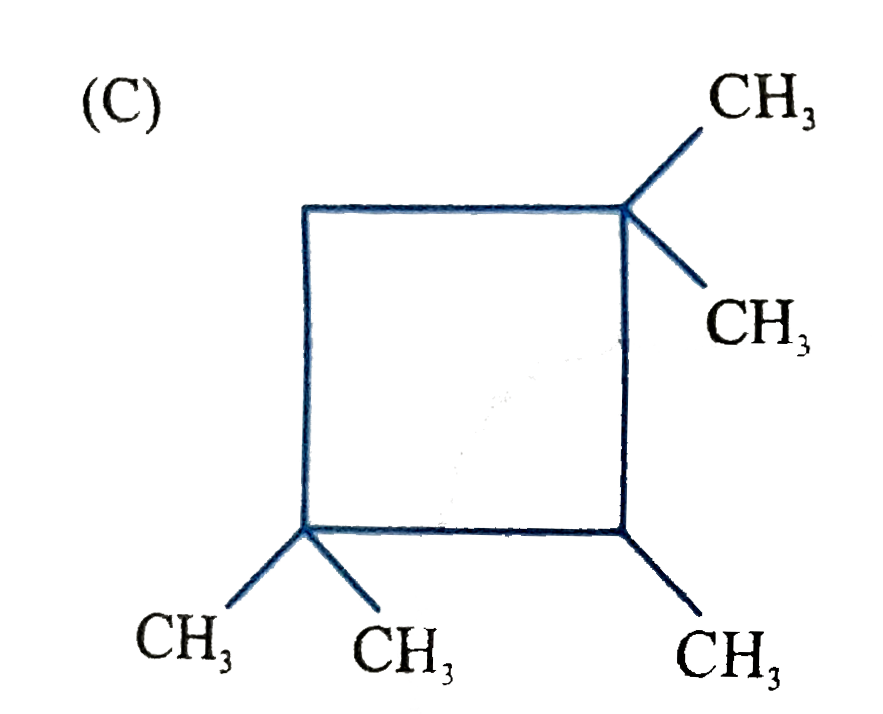
C
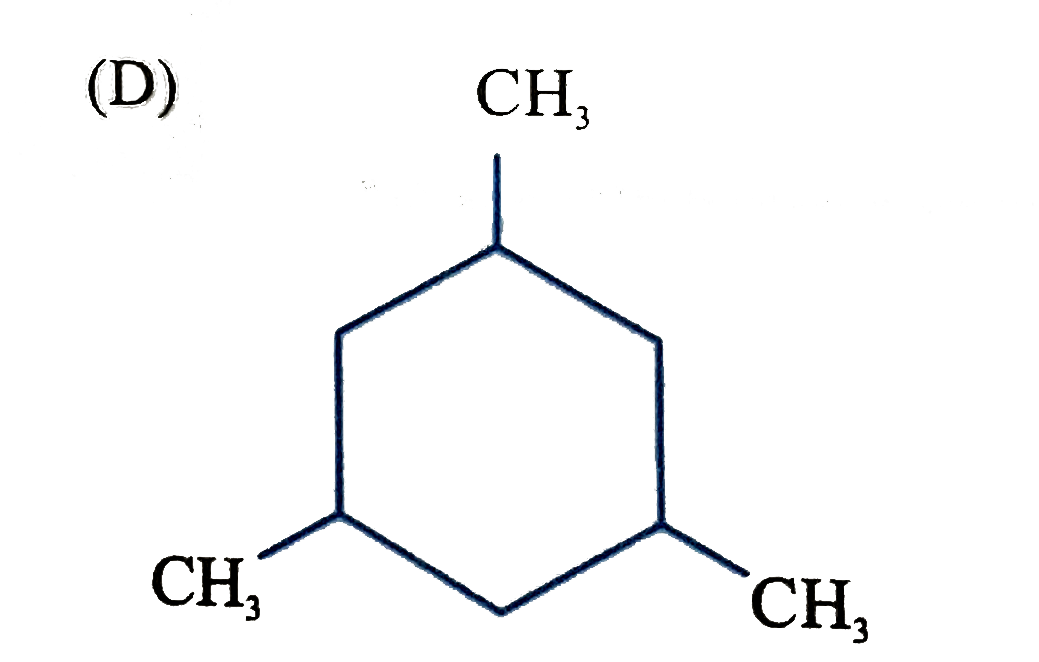
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
NARAYNA|Exercise II) Keto-Enoltautomerism:|7 VideosGENERAL ORGANIC CHEMISTRY
NARAYNA|Exercise Geometrical Isomerism:|33 VideosGENERAL ORGANIC CHEMISTRY
NARAYNA|Exercise Level II|17 VideosGENERAL ORGANIC CHEMISTRY
NARAYNA|Exercise LEVEL-II (H.W)|21 VideosHYDROCARBONS
NARAYNA|Exercise EXERCISE - 4|18 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-GENERAL ORGANIC CHEMISTRY-Level IV Ncert based questions I) Structural isomerism:
- How many isomeric (excluding stereo) carboxylic acids are possible for...
Text Solution
|
- Minimum no. of carbon atoms required for an alkane to exhibit position...
Text Solution
|
- How many secondary amines are possible for C(5)H(13)N
Text Solution
|
- An organic compound has molecular formula C(9)H(18). It is a saturated...
Text Solution
|
- Which compound is not the isomer of 3-Ethyle-2- methylpentane?
Text Solution
|
- Which of the following is not an isomer of CH(3)-CH(2)CHO
Text Solution
|