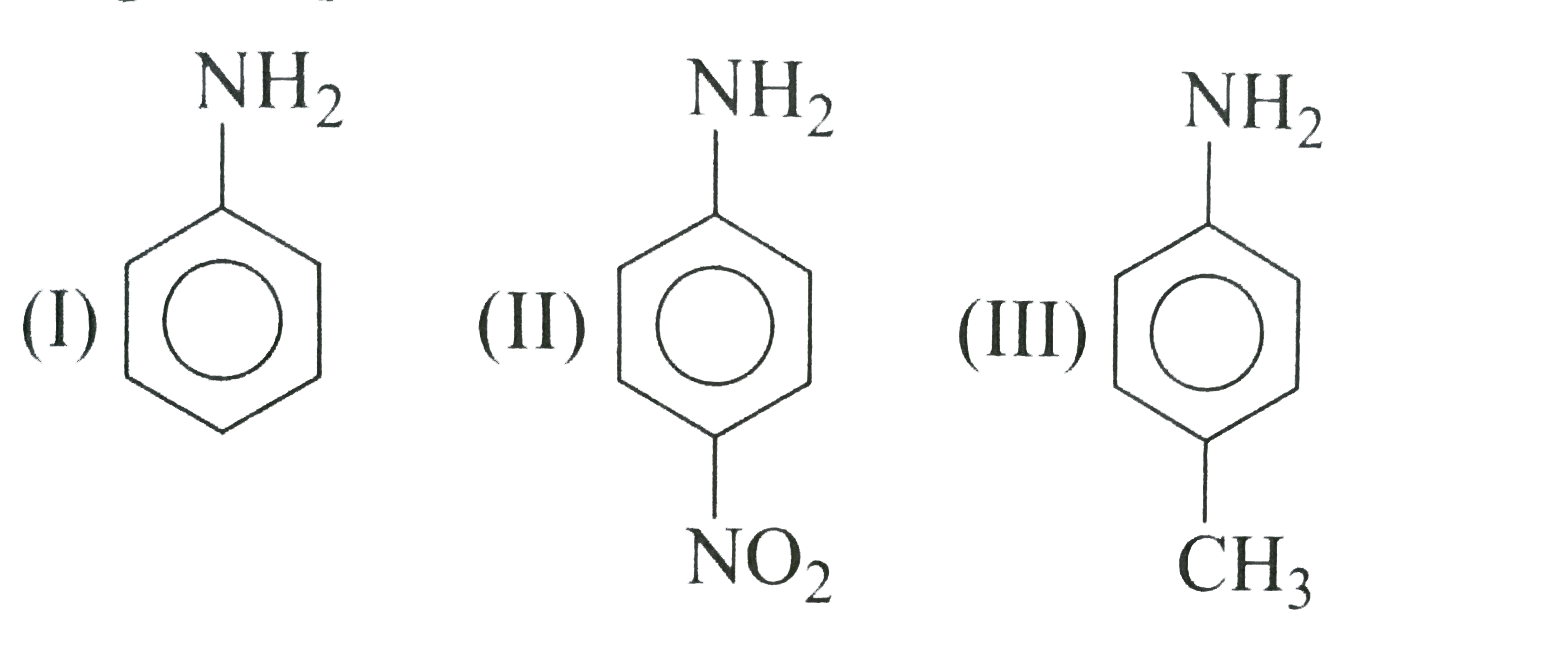
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-NITROGEN CONTAINING COMPOUNDS-BITSAT Archives
- The correct increasing order of basic strength for the following compo...
Text Solution
|
- Arrange the following in correct order of basicity
Text Solution
|
- C(6)H(5)NH(2)underset(180^(@)C)overset(H(2)SO(4))rarrunderset("Para fo...
Text Solution
|
- Identify C in the following reaction:
Text Solution
|
- The structure of the compound formed, when nitrobenzene is reduced by ...
Text Solution
|
- The IUPAC name of the compound, underset(OH)underset(|)(CH(2))-underse...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Aniline reacts with conc. HNO(3) to give
Text Solution
|