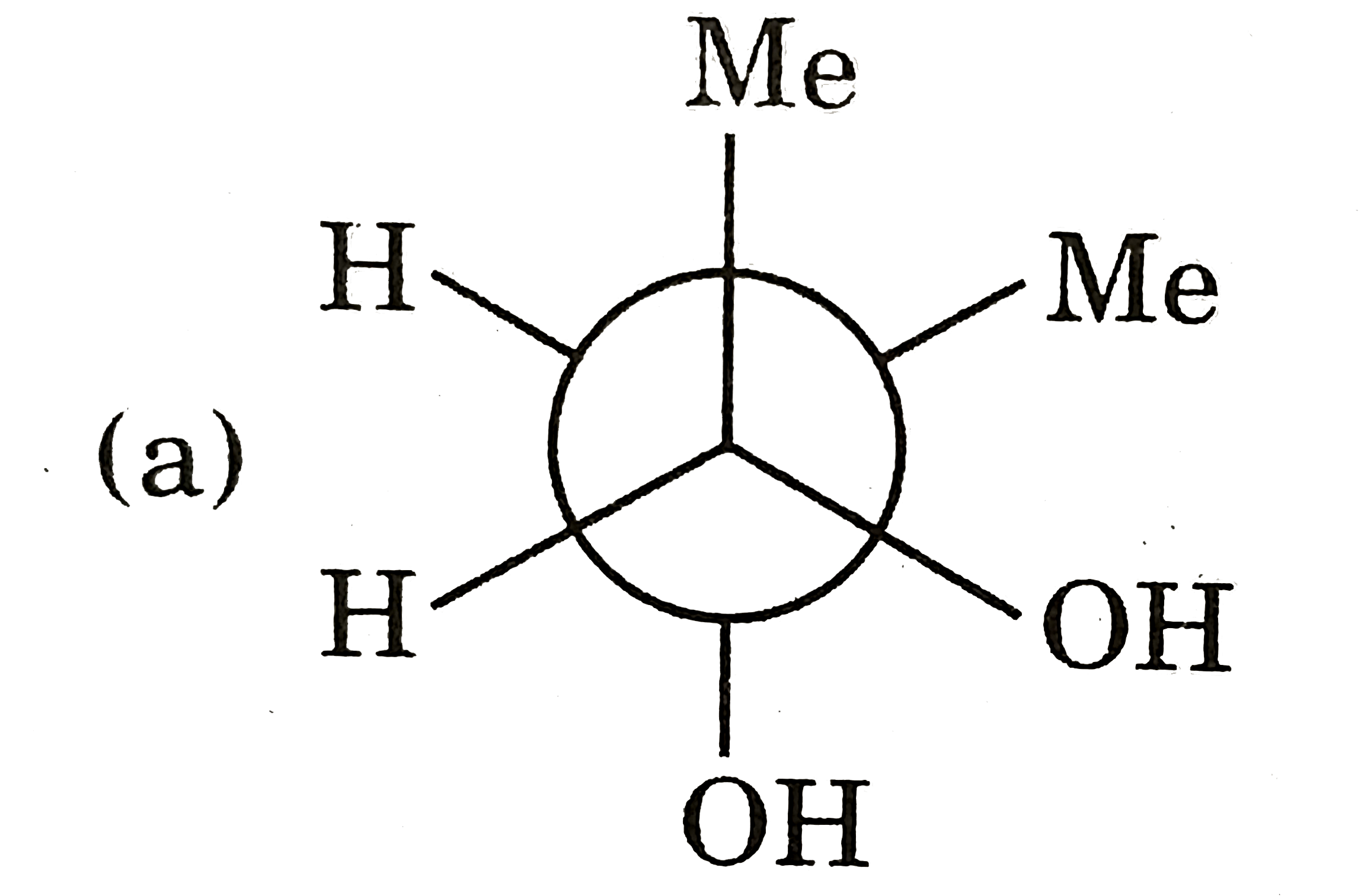
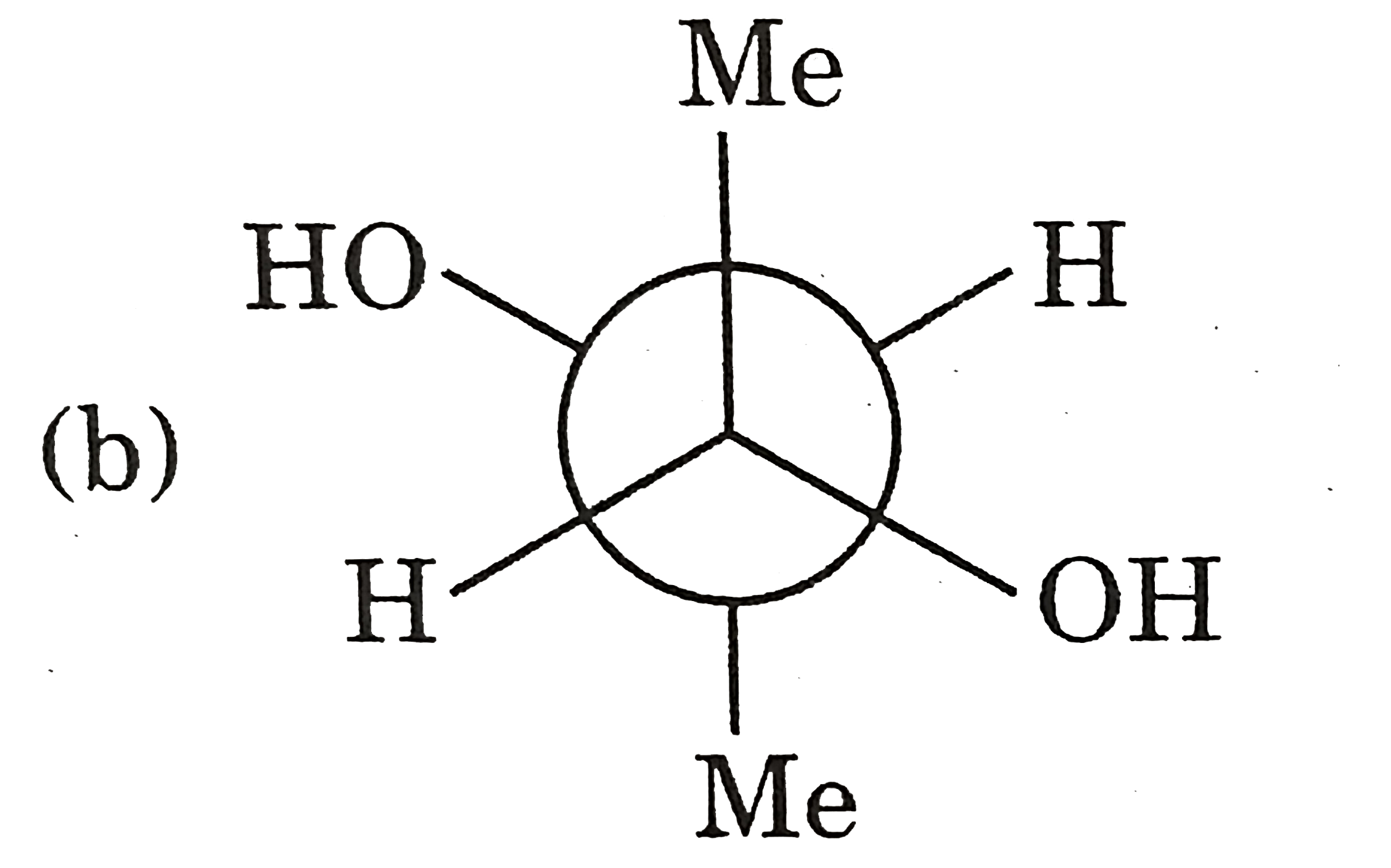
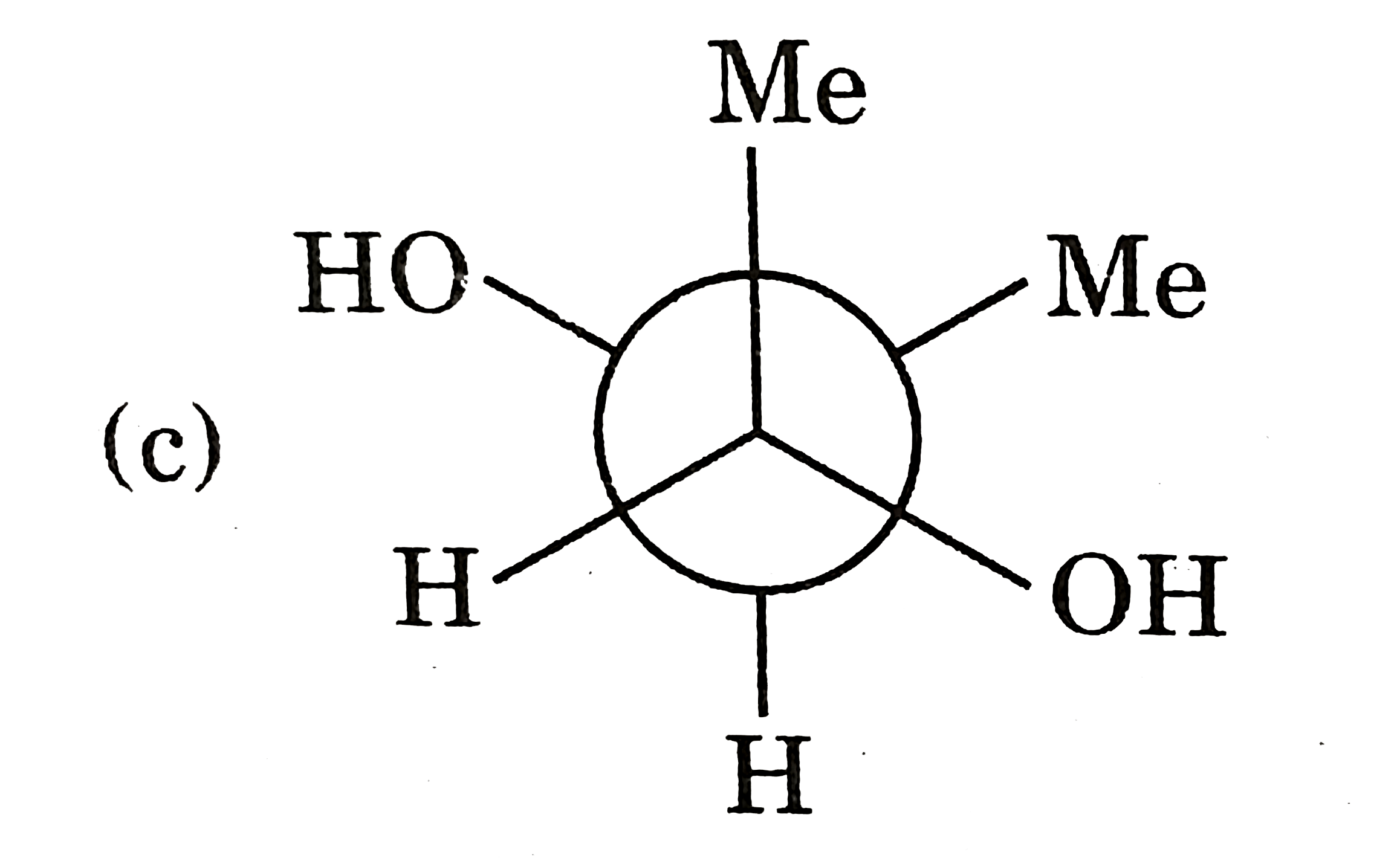
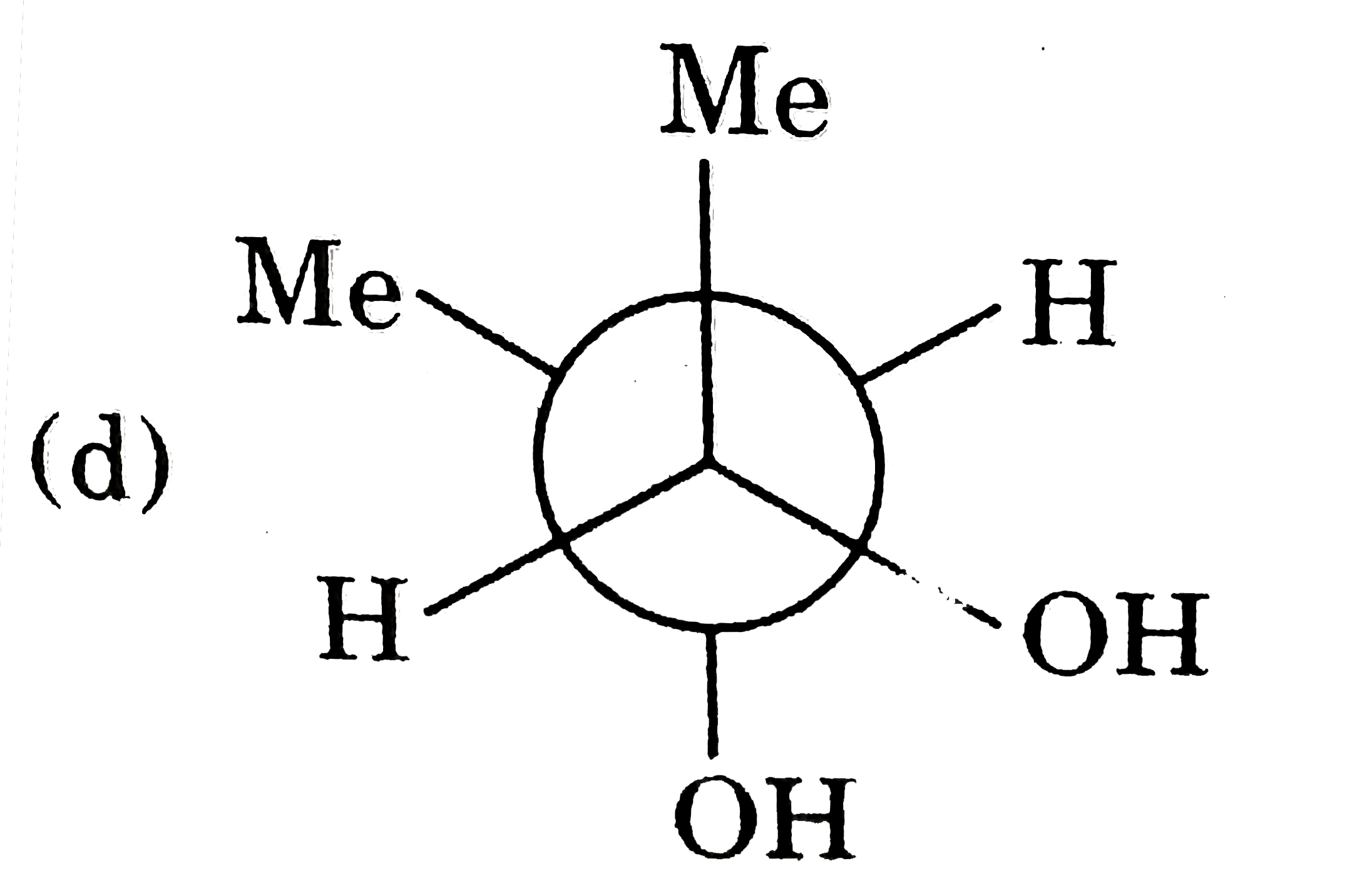
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ISOMERISM-SUBJECTIVE TYPE
- Most stable from of meso-2,3-butandiol is :
Text Solution
|
- Total nubler of mono chlorodervatives obrained by replacement of H by ...
Text Solution
|
- Calculate valur of x+y+z+if xto Number of chiral centre. yto Numb...
Text Solution
|
- Tetracyline is called a broad spectrum antibiontic because it active a...
Text Solution
|
- How many stereoismers are possible for the following compound?
Text Solution
|
- What will be the number of hydrogen atoms (Y) replaced by D on prolong...
Text Solution
|
- underset(hv)overset(Cl(2))rarr number of theretically possible dichlor...
Text Solution
|
- Total number of stereoisomer in given compound are : CH(3)-overset(O...
Text Solution
|
- Cyclohexane-4,4-idone is a polar compound, having dipole moment value ...
Text Solution
|
- How many H are replaced by D on prolonged treament in the following? ...
Text Solution
|
- Number of chiral centres present in above compound. Number of teoret...
Text Solution
|
- When 20 gm optically active compound is placed in a 10 dm tube, in a 2...
Text Solution
|
- How many plane of symmetry are present in cyclopropane?
Text Solution
|
- How many chiral carbon atoms are present in the following compound?
Text Solution
|
- How many plane of symmetry are present in prismane (C(6)H(6))?
Text Solution
|
- An unknown compound weighin 4.2 gm is dissolved in enotgh carbon tetra...
Text Solution
|
- How many compounds are theretically possible for formula (C(6)H(6)O) (...
Text Solution
|
- How many of the following are resolvable ?
Text Solution
|
- How many total number of structural isomers are possible for C(4)H(7)C...
Text Solution
|
- How many total number of structural isomers of C(4)H(6)Cl(2) are possi...
Text Solution
|
- Dipole moment of a compound W-CH(2)-CH(2)-W is 1.5 D.if dipole moment ...
Text Solution
|