A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
GRB PUBLICATION|Exercise MULTIPLE OBJECTIVE TYPE|71 VideosISOMERISM
GRB PUBLICATION|Exercise COMPREHENSION TYPE|25 VideosISOMERISM
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|67 VideosGASEOUS STATE
GRB PUBLICATION|Exercise Exercise|530 VideosMOLE CONCEPT, STOICHIOMETRY & CONCENTRATION TERMS
GRB PUBLICATION|Exercise Subjective type|145 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ISOMERISM-REASONING TYPE
- Statement-1: Me-C= and Me-oversetrarrN=C are not functional isomers. ...
Text Solution
|
- Statement I: 5,5-dimethyl-1,3-cyclohexanedione exists predominantely i...
Text Solution
|
- Statement I: Resonating structures of can show geometrical isomerism ...
Text Solution
|
- Stetement-1: Boat fromis the least stable conformation of cyclohexane....
Text Solution
|
- Statement-1: is more stable Statement-2: Torsional strain and flag ...
Text Solution
|
- STATEMENT-1: In aqueous medium gauch from is the most stable from of H...
Text Solution
|
- STATEMENT-1: contain benzenoid structure. STATEMENT-2: contain be...
Text Solution
|
- STATEMENT-2: Structural isomer having same type of carbon chain but di...
Text Solution
|
- STATEMENT-1: ##GRBORGCHMV02QBC03E01149Q02.png" width="80%">
Text Solution
|
- STATEMENT-1: STATEMENT-2: Molecules containing plane of symmetry or...
Text Solution
|
- STATEMENT-1: (A) is optically active and (B) is optically inactive. ...
Text Solution
|
- STATEMENT-1: Cyclohexen shows gemetrical siomerism. STATEMENT-2: Its...
Text Solution
|
- STATEMENT-1: STATEMENT-2: No symmetry element is present in above c...
Text Solution
|
- STATEMENT-1: Disteremers are morror image of each other. STATEMENT-2...
Text Solution
|
- STATEMENT-1: STATEMENT-2: There is no chiral carbon inthis compound...
Text Solution
|
- STATEMENT-1: 2-butanol is a chiral molecule. STATEMETN-2: 2-butanol ...
Text Solution
|
- STATEMENT-1: STATEMENT-2:
Text Solution
|
- STATEMENT -1: 2,3-dimethyl butane is a meso compound. STATEMENT-2: I...
Text Solution
|
- STATEMENT-1: is non-resolvable. STATEMENT-2: There is no chiral car...
Text Solution
|
- Assertion (A): The cis form of (CH(3)-underset(Cl)underset(|)(CH)-C...
Text Solution
|
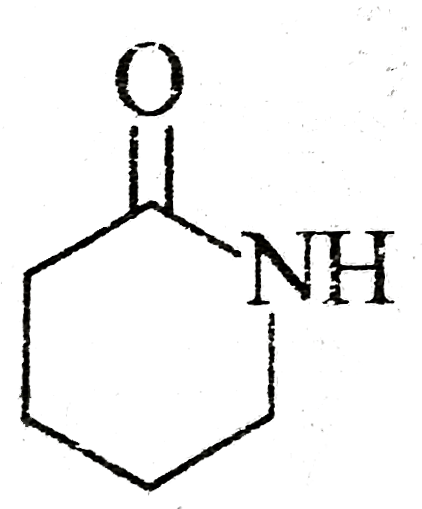 can show geometrical isomerism at room temperature
can show geometrical isomerism at room temperature