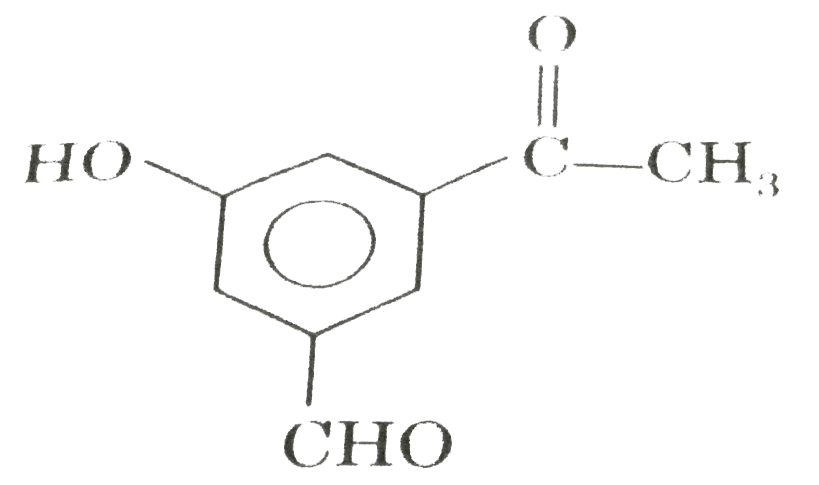
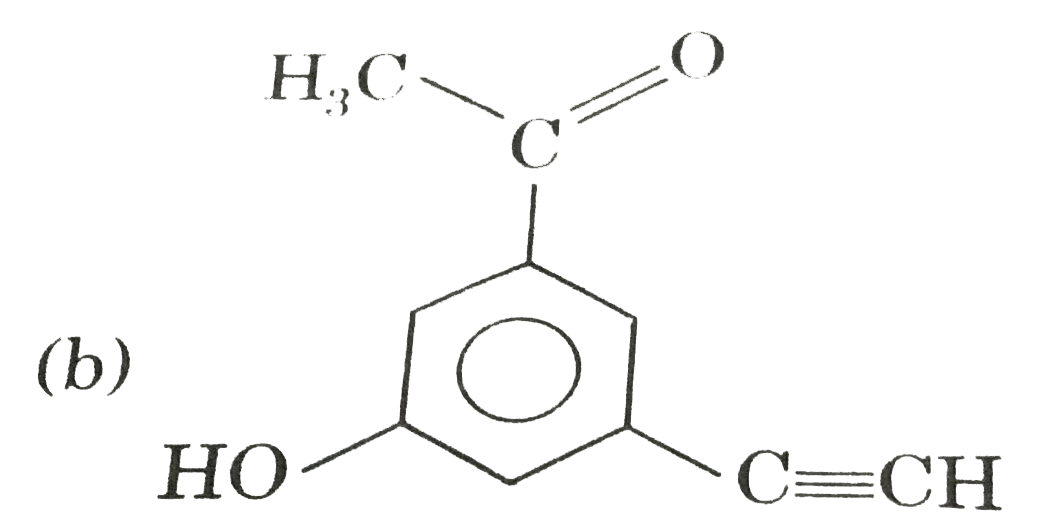
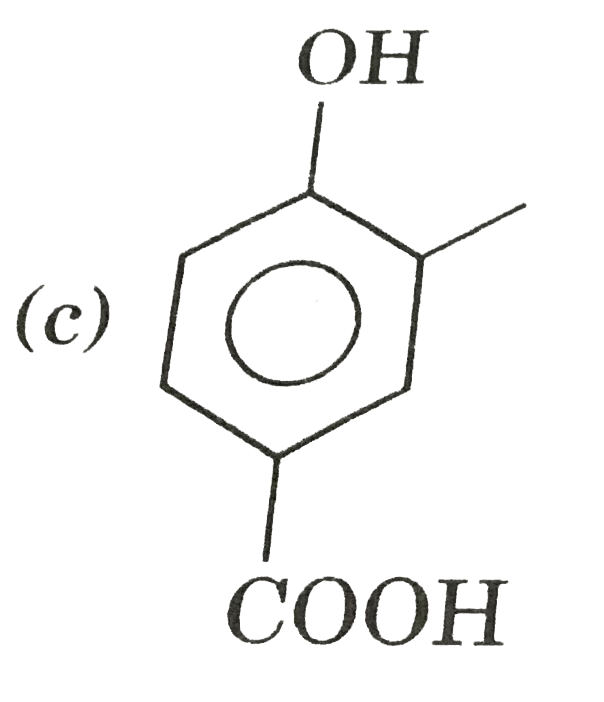
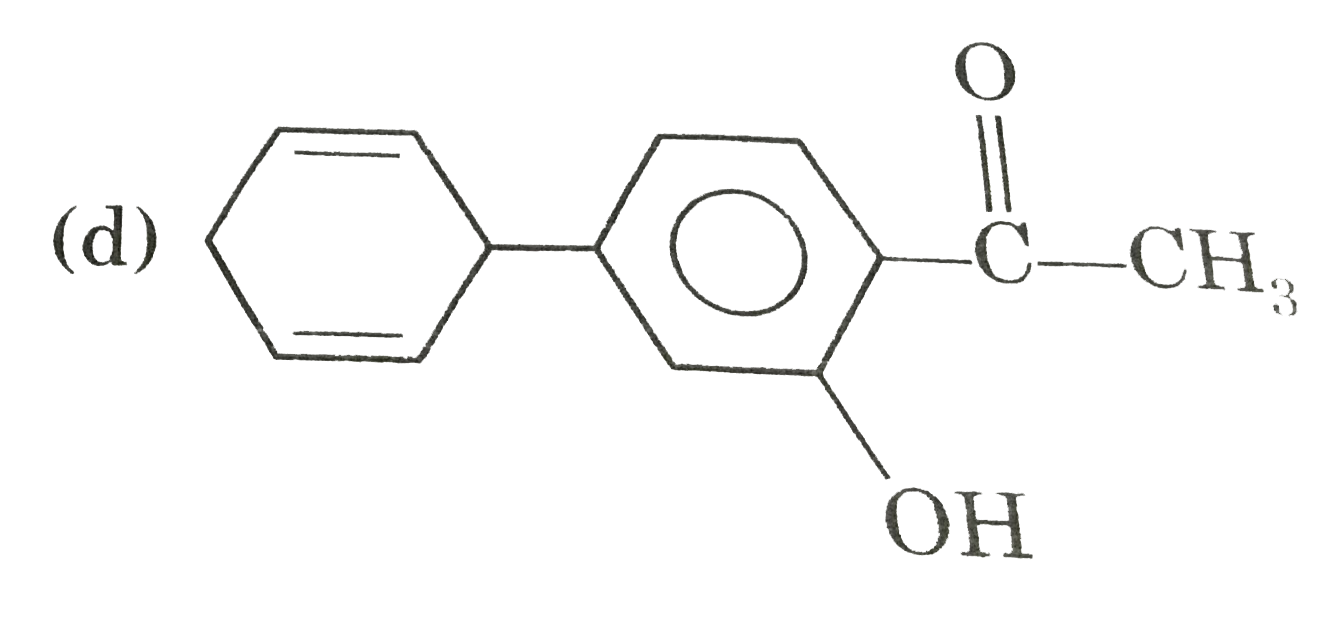
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFE
GRB PUBLICATION|Exercise REASONING TYPE|27 VideosBIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFE
GRB PUBLICATION|Exercise MULTIPLE OBJECTIVE TYPE|72 VideosALCOHOLS AND ETHERS
GRB PUBLICATION|Exercise level 62|1 VideosCARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDS
GRB PUBLICATION|Exercise Subjective type|19 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-BIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFE-SUBJECTIVE TYPE
- A copound which can give iodoform test , bromine water test ,FeCl(3)te...
Text Solution
|
- Glycin has the structural formula NH(2)-CH(2)-COOH. Glycine has two di...
Text Solution
|
- Glucose + Fructose + Monnose underset("(Excess)")overset(PhNHNH(2))ra...
Text Solution
|
- Consider following carbohydrates. alpha-D-glucose,beta-D-fructose, Ma...
Text Solution
|
- Number of dipeptide which can be formed by : Glycine, Alanine, Leucine...
Text Solution
|
- overset(+)NH(3)-CH®-CO(2)H has its Ka(1)=10^(-7). The isoelectric poi...
Text Solution
|
- How many reducing saccharides are there in following list ? (a) alp...
Text Solution
|
- How many of following compounds will give red colour with Barfoed reag...
Text Solution
|
- How many essential amino acids are there in following list ? (a) Gly...
Text Solution
|
- Atetrapeptide on complete hydrolysis gave Alanine, Glycine, Leucine an...
Text Solution
|
- Count water insoluble vatamins out of following : (a) Vitamin A (b...
Text Solution
|
- Per monomeric unit, how much is the increase in mass when cellulose is...
Text Solution
|
- Smallest carbohydrate contain how many carbon atoms ?
Text Solution
|
- Glucoseoverset(HCN)rarrAunderset(pdBaSO(4))overset(H(2))rarrBoverset(H...
Text Solution
|
- Glucose overset(NH(2)OH)rarrAunderset("excess")overset(AcOAc)rarrBunde...
Text Solution
|
- Number of mole of HIO(4) required per mol for complatr reaction of alp...
Text Solution
|
- How many amino acids migrates towards anode on electrolysis at pH = 8 ...
Text Solution
|
- Consider following polmers : Polythene, PVC, Bskelite, Nylon-6,6, Da...
Text Solution
|
- Out of following number of aplymers which are formed by condensation ...
Text Solution
|
- Select the given code of reagents for following canversion and write y...
Text Solution
|
- Number of pi electrons which are delocalized in melamine is X and numb...
Text Solution
|