The Grignard reagent is a very powerful base, in effect it contains a carbanion. Thus, it is not possible to prepare a Grignard reagent from an group that contains an acidic hydrogen , by an acidic hydrogen, we mean any hydrogen more acidic than the hydrogen atoms of an alkane or alkene. We cannot, for example, prepare a Grignard reagent from a compound containing an -OH group, an `-NH-` group, an -SH group, a `-CO_(2)H` group, or an `-SO_(3)H` group. If we were to attempt to prepare a Grignard reagent from an organic halide containing any of these groups, the formation of the Grignard reagent would simply fail to take place. (Even if a Grignard reagent were to form, it would immediately react with the acidic group.)
Since Grignard reagents are powerful nucleophiles, we cannot prepare a Grignard reagent from any organic halide that contains a carbonyl, epoxy, nitro, or cyano (-CN) group. If we were to attempt to carry out this kind of reaction, any Grignard reagent that formed would only react with the unreacted starting material:
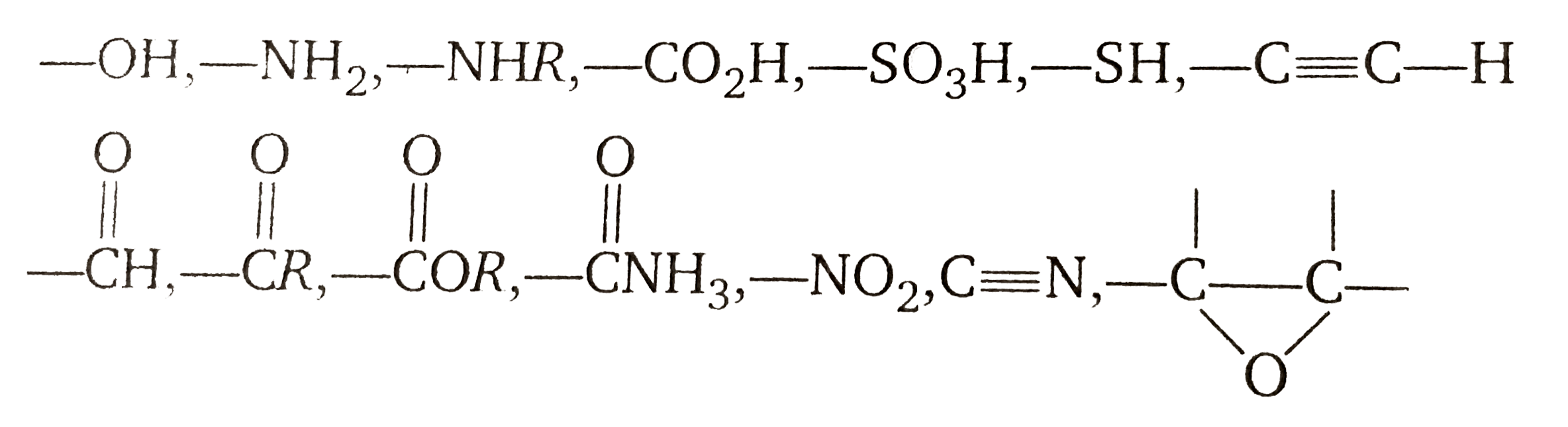
This means that when we prepare Grignard reagents, we are effectively limited to alkyl halides or to analogous organic halides containing carbon-carbon douvle bonds, internal triple bonds, ether linkages, and `-NR_(2)` groups.