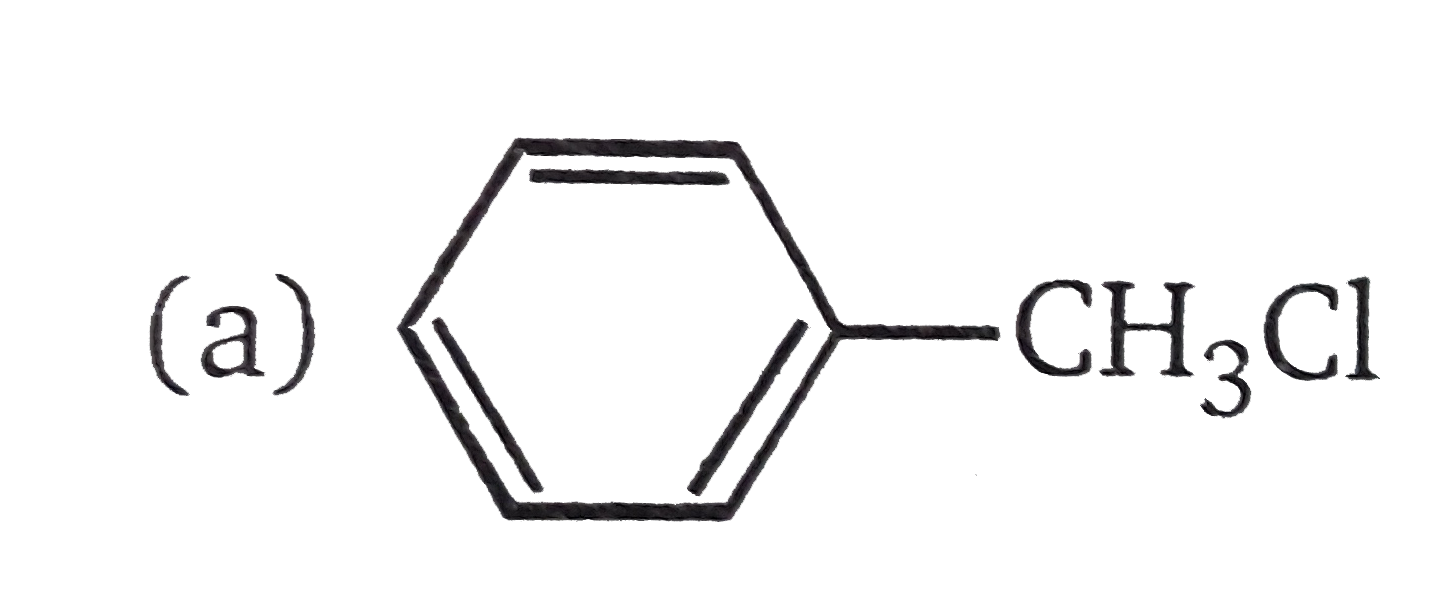
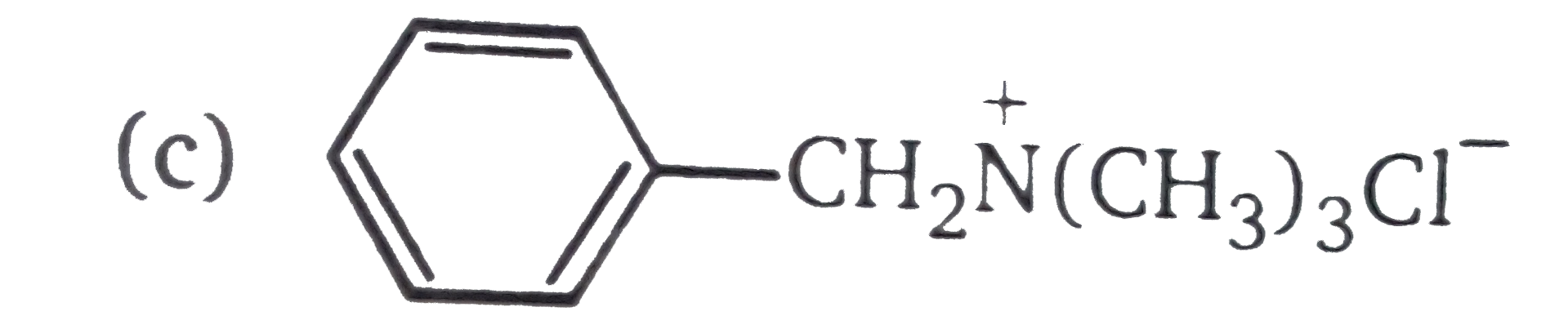
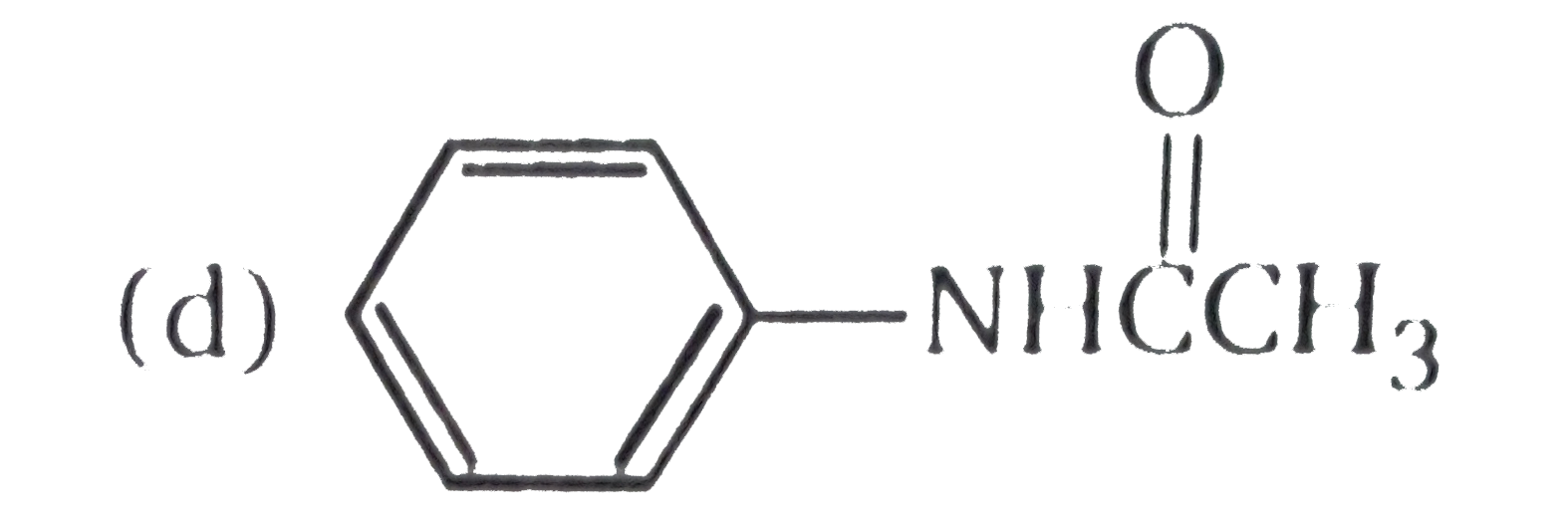
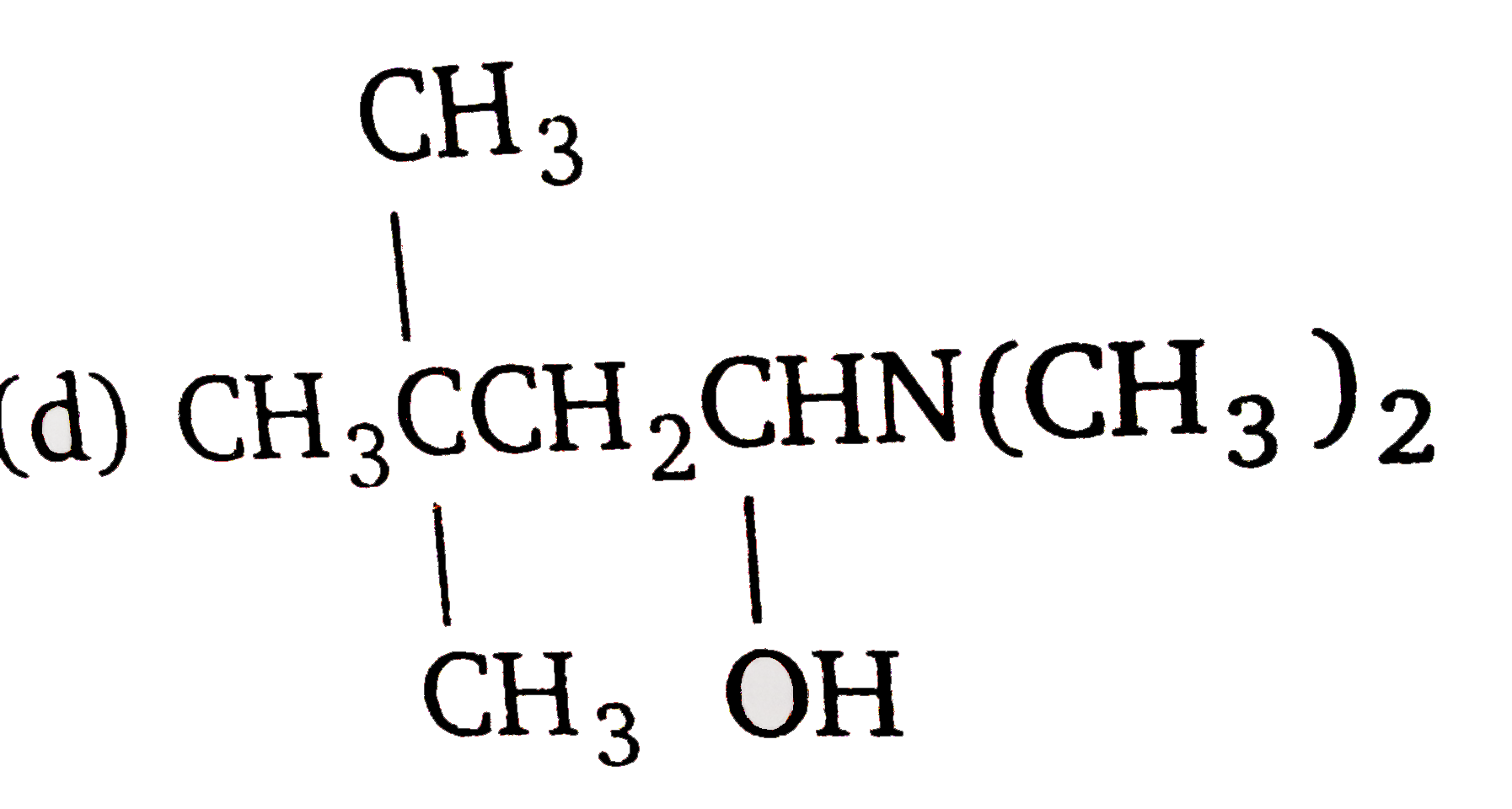A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AMINES
MS CHOUHAN|Exercise Level 2|1 VideosAMINES
MS CHOUHAN|Exercise SOLVED PROBLEM|5 VideosALKENES AND ALKYNES I
MS CHOUHAN|Exercise Additional Objective Questions (Multiple Correct Choice Type)|2 VideosAMINO ACIDS AND PROTEINS
MS CHOUHAN|Exercise Additional Objective Questions (Integer Type)|1 Videos
Similar Questions
Explore conceptually related problems
MS CHOUHAN-AMINES-SINGLE CORRECT CHOICE TYPE
- Which one of the following is best catalyst for the reaction shown bel...
Text Solution
|
- Acetamide is treated separately with the following reagents. Which of ...
Text Solution
|
- Best method for preparing primary amines from alkyl halides without ch...
Text Solution
|
- The Gabriel synthesis is a method to generate primary
Text Solution
|
- An organic compound A upon reacting with NH(3) gives B. On heating, B ...
Text Solution
|
- Which one of the following methods is neither meant for the synthesis ...
Text Solution
|
- Which of the following diazonium salts cannot be isolated?
Text Solution
|
- Which amine yields N-nitroso amine after treatment with nitrous acid?
Text Solution
|
- Amongst the compounds given, the one that would form a brilliant color...
Text Solution
|
- The nitrogen atom in the following cyclic compounds can be removed as ...
Text Solution
|
- What will be the final product of the reaction of cyclohexanamine with...
Text Solution
|
- Which of the following compound is the strongest base?
Text Solution
|
- Which of the following is least reactive toward S(N)Ar?
Text Solution
|