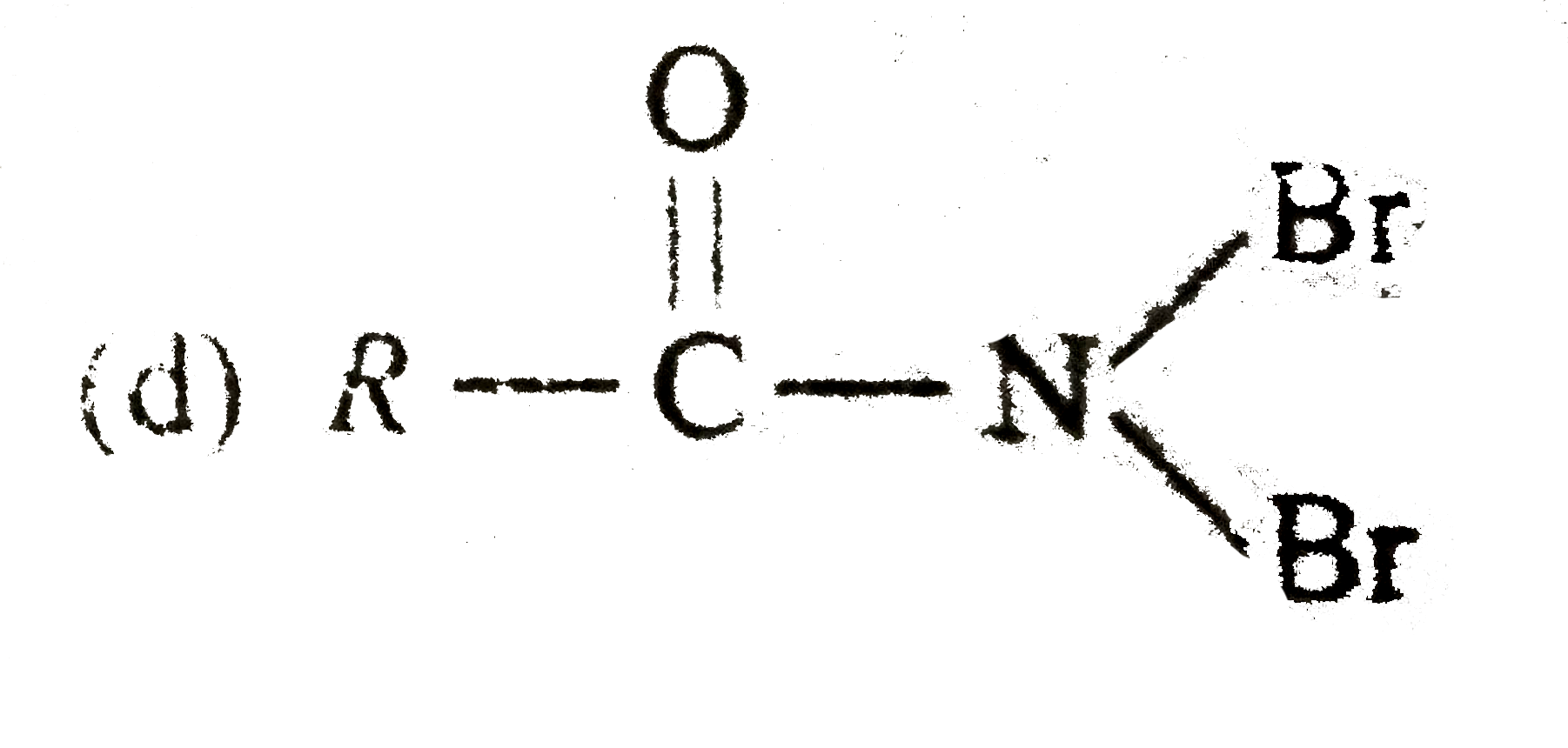A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-AMINES-Subjective Type Problems
- Reaction of RCONH(2) with a mixture of Br(2) and KOH gives RNH(2) as t...
Text Solution
|
- Find out finla products of following reactions:
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|