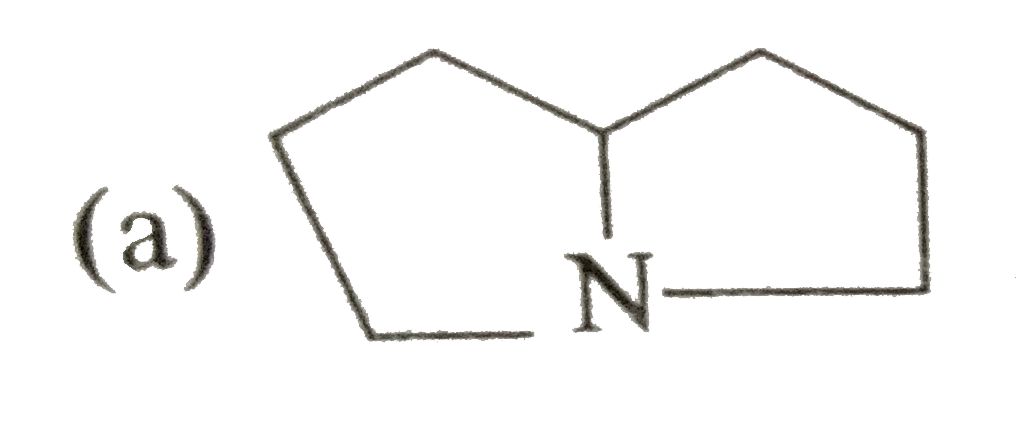
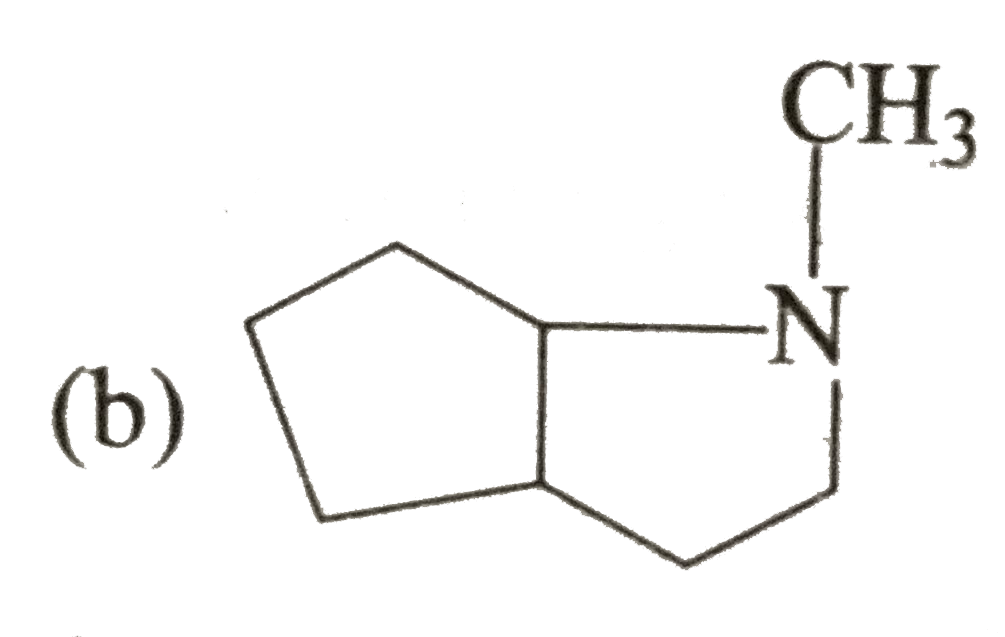
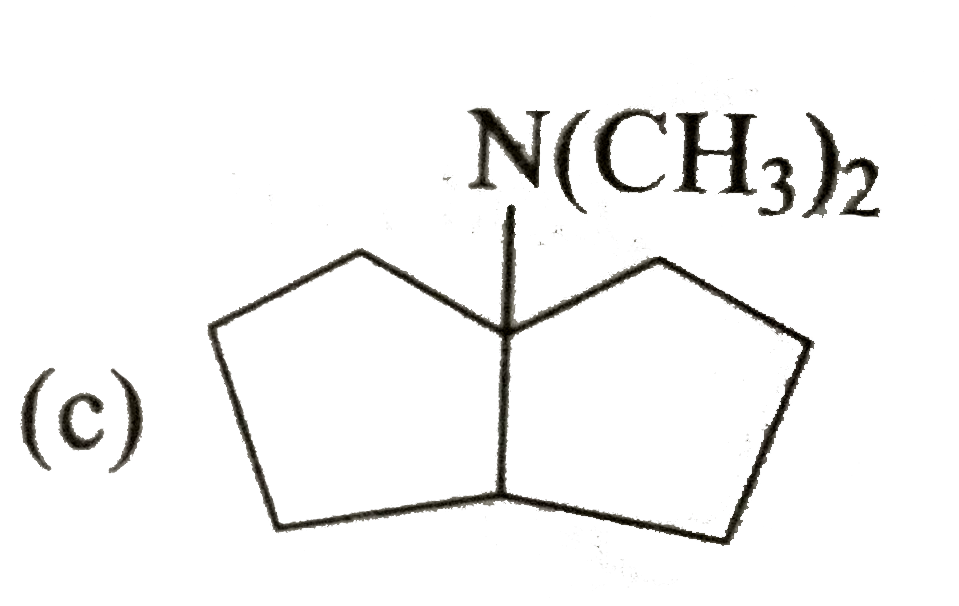
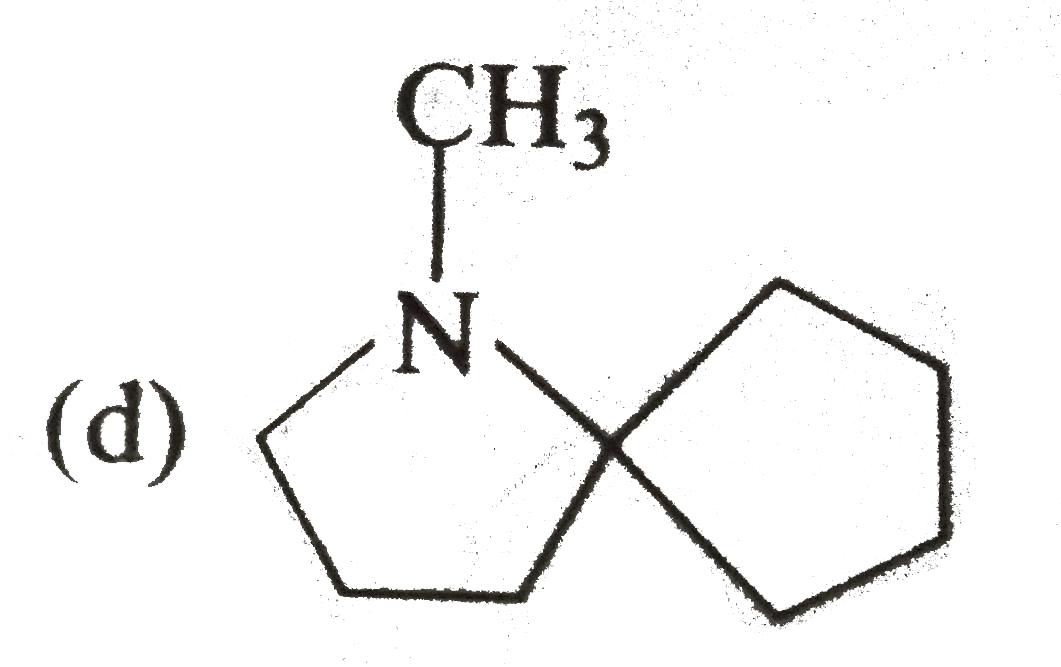
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AMINES
HIMANSHU PANDEY|Exercise Level 2 (Q.51 To Q.75)|25 VideosAMINES
HIMANSHU PANDEY|Exercise Level 2 (Q.76 To Q.89)|14 VideosAMINES
HIMANSHU PANDEY|Exercise Level 2 (Q.1 To Q.25)|25 VideosALCOHOLS AND ETHERS
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Subjective Type Problems|9 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-AMINES-Level 2 (Q.26 To Q.50)
- Which of the following is the strongest Bronsted base?
Text Solution
|
- Which of the following is the weakest Bronsted base?
Text Solution
|
- Which of the following is stronger Bronsted base?
Text Solution
|
- For the following compounds, which is the strongest base and which is ...
Text Solution
|
- Which compound is the likely from following reaction?
Text Solution
|
- Which of these is the strongest base?
Text Solution
|
- What sequence of reaction would best accomplish the following reaction...
Text Solution
|
- What is the likely product from the following reaction?
Text Solution
|
- Repeated Hofmann elimination reaction (exhaustive methylation followed...
Text Solution
|
- Only one of the following amines will lose its nitrogen atom as trimet...
Text Solution
|
- The nitrogan atom in each of the following tertiary amines may be remo...
Text Solution
|
- The Hinsberg test of a C(5)H(14)N(2) compound produces a solid that i...
Text Solution
|
- What set of conditions would be useful for preparing a 2^(@) amine?
Text Solution
|
- Which of the following amines reacts most rapidly with
Text Solution
|
- Consider the following sequence of reactions: Identify product C:
Text Solution
|
- The major product formed in the reaction is:
Text Solution
|
- The product formed in the reaction is :
Text Solution
|
- Identify B:
Text Solution
|
- The final product (B) is:
Text Solution
|
- The final major product of the reaction is: Ph-overset(OH)overset(|)...
Text Solution
|