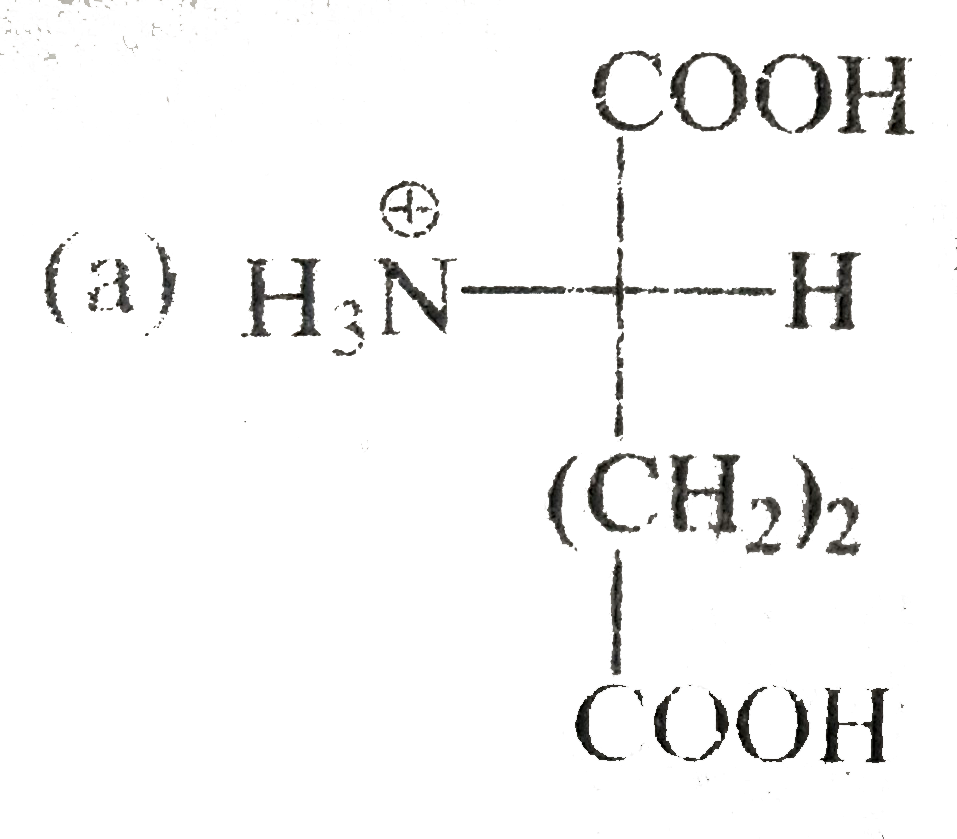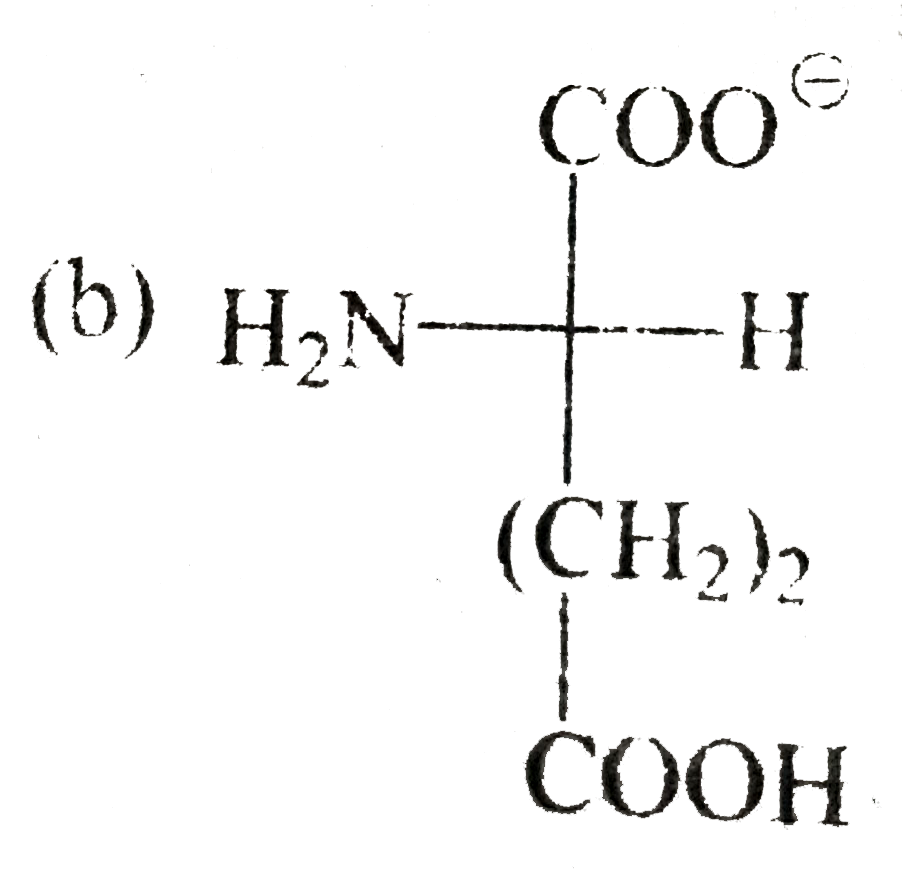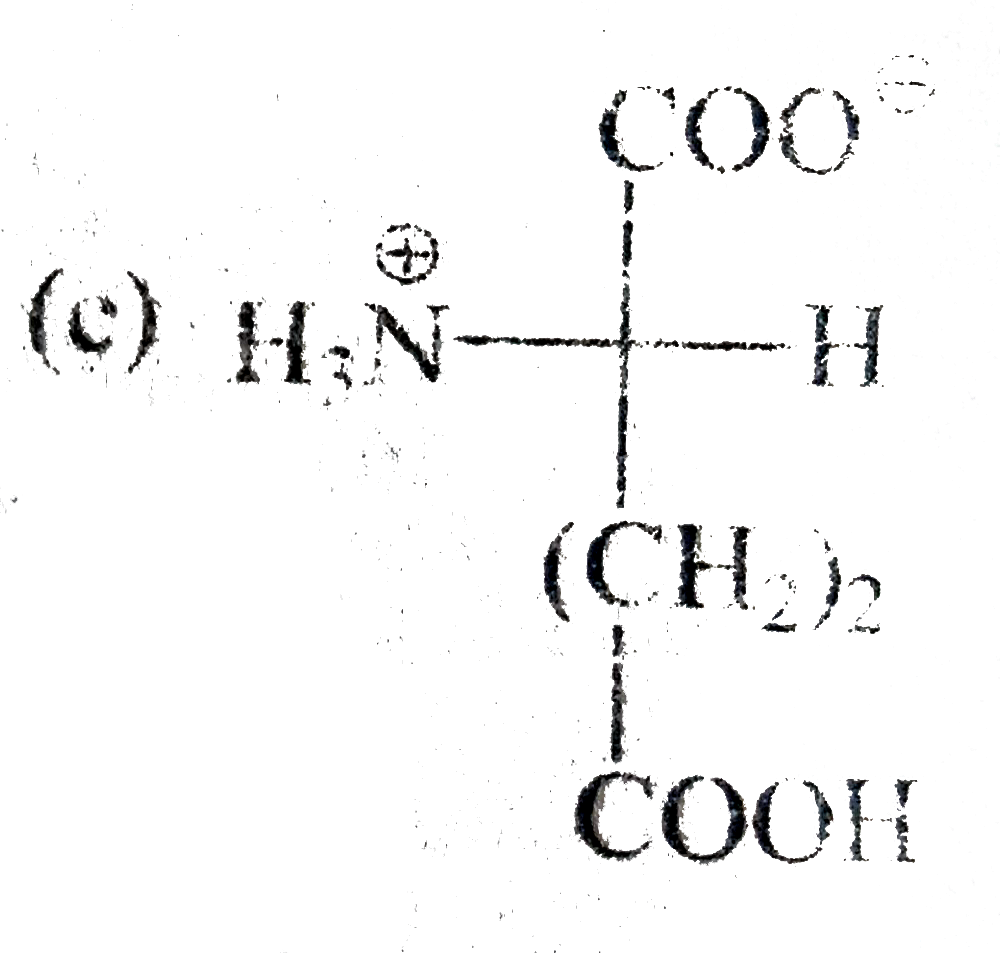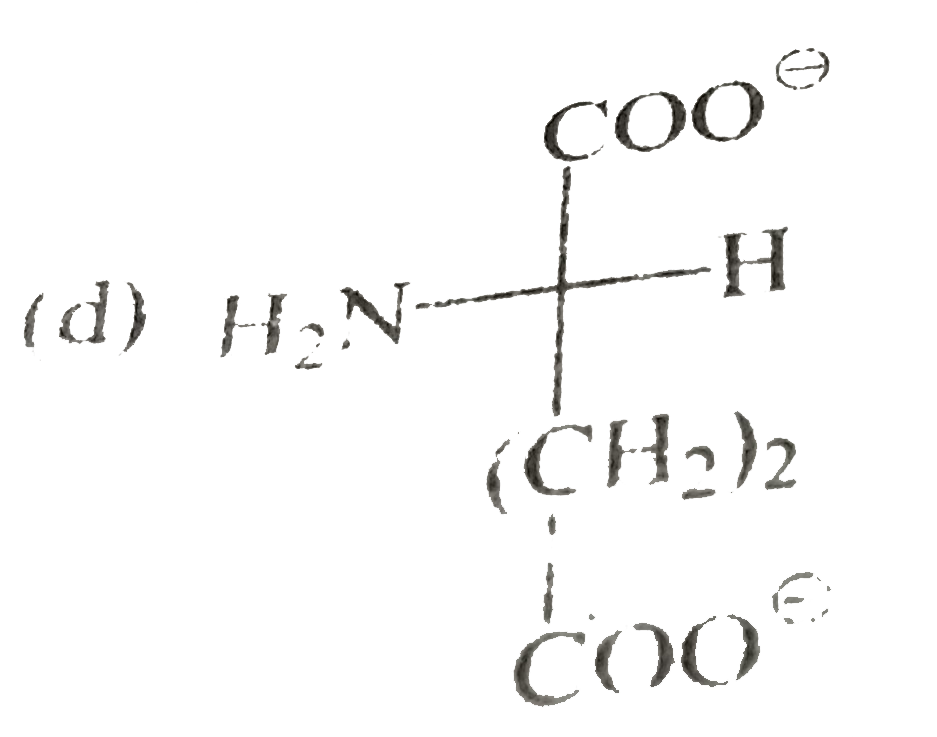A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES
HIMANSHU PANDEY|Exercise Level 2 (Q.51 To Q.55)|5 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise More Than One Correct|23 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise Level 2 (Q.1 To Q.25)|25 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Subjective Type Problems|9 VideosCABOXYLIC ACIDS AND ITS DERIVATIVES
HIMANSHU PANDEY|Exercise Subjective Type Problems|8 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-BIOMOLECULES-Level 2 (Q.26 To Q.50)
- Which of the following represents the anomer of the compound shown ?
Text Solution
|
- , the given is enol form of :
Text Solution
|
- The number of chiral centers in glucopyranose and fructofuranose are:
Text Solution
|
- Which of the following is an amino acid?
Text Solution
|
- Amino acids udergo internal acid base reaction to form:
Text Solution
|
- An amino acid usually shows its lowest solubility in water:
Text Solution
|
- Which one among following is a peptide linkage?
Text Solution
|
- Consider the following sequence of reaction, overset(1. BrCH(2)(CO...
Text Solution
|
- Which of the following is the major solute species in a solution of ly...
Text Solution
|
- Which of the following is the major solute species in a solution of gl...
Text Solution
|
- Which of the following statements most correctly defines the isoelectr...
Text Solution
|
- Alanine at its isoelectric point, exist in solution as :
Text Solution
|
- The order of decreasing acidity of these acidic sites is :
Text Solution
|
- Biuret test is used for the detection of :
Text Solution
|
- alpha-Amino acids behave as crystalline ionic solid and have high melt...
Text Solution
|
- Which of the following is correct structure of histidine at pH=0?
Text Solution
|
- Find out final produt:
Text Solution
|
- Match the following columns
Text Solution
|
Text Solution
|
- Find out the structure of lactose:
Text Solution
|