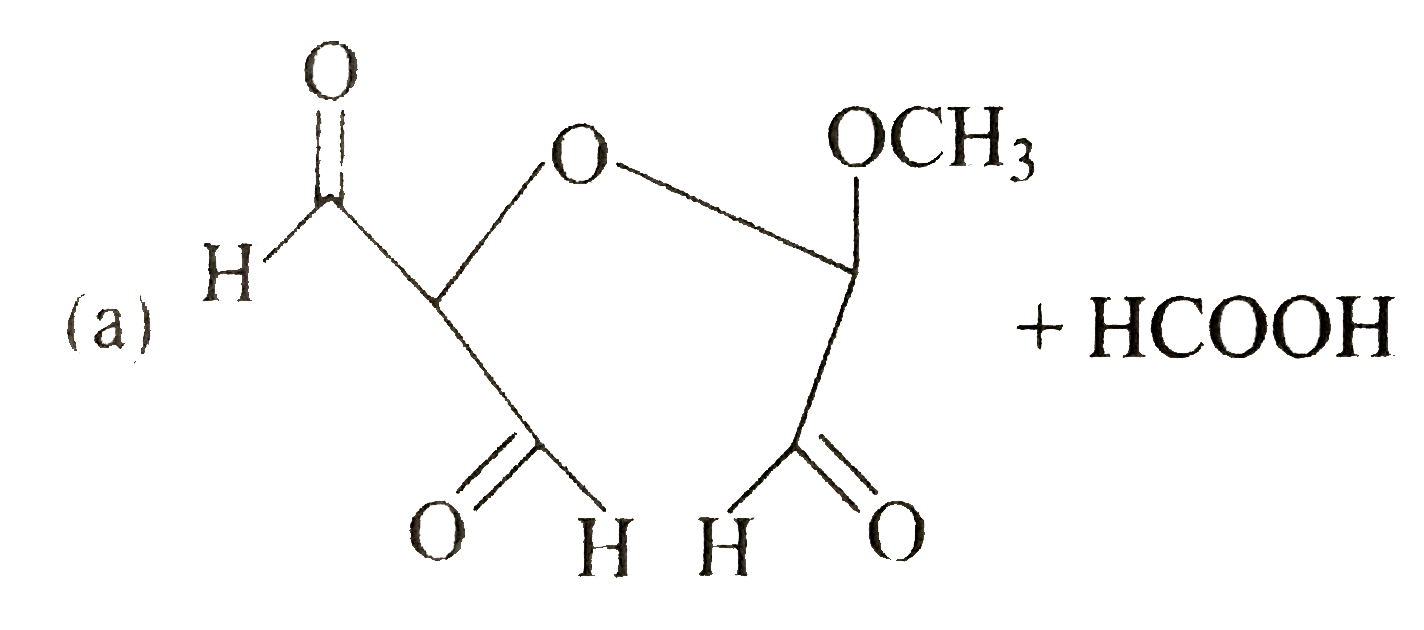
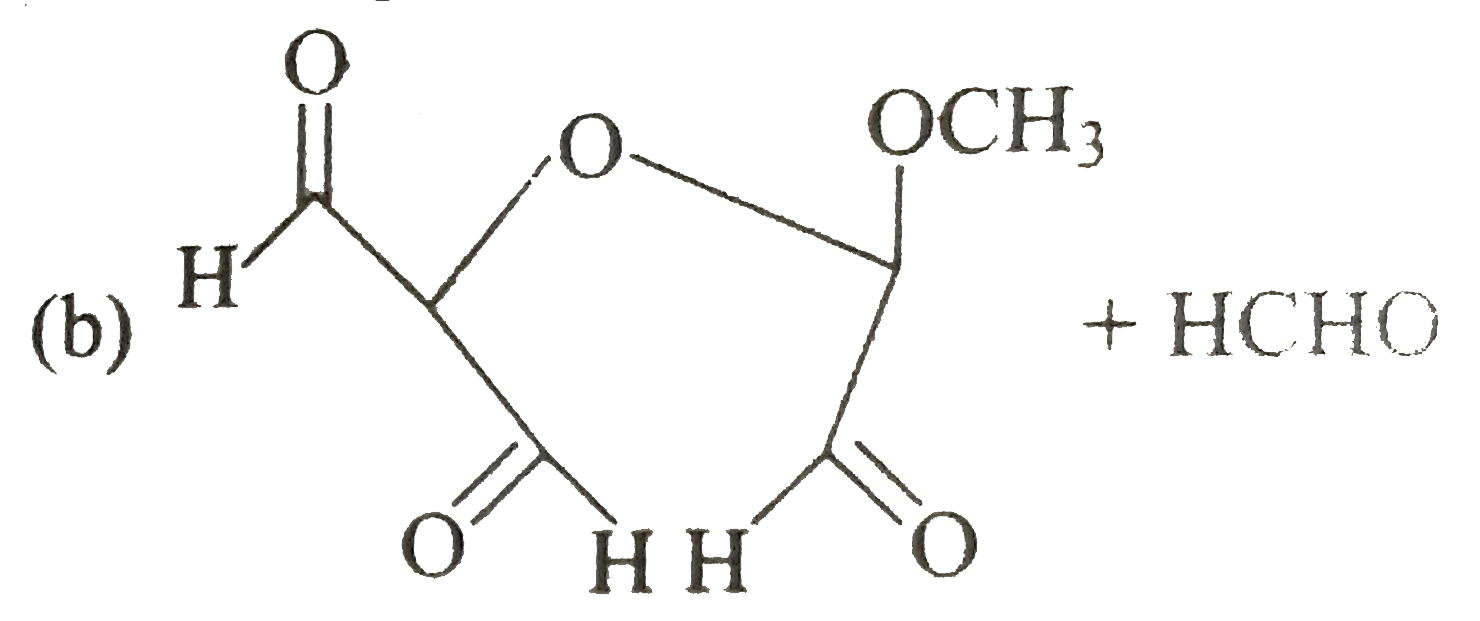
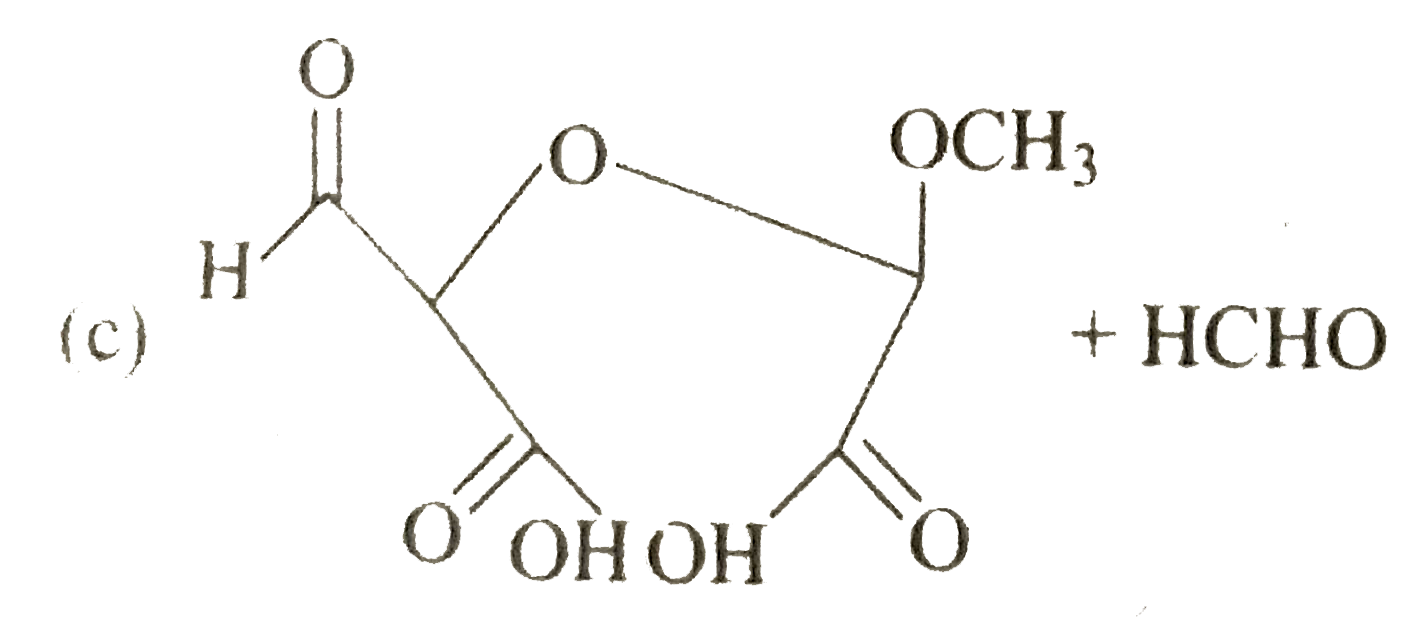
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
BIOMOLECULES
HIMANSHU PANDEY|Exercise More Than One Correct|23 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise Linked Comprehension Type|12 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise Level 2 (Q.26 To Q.50)|25 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Subjective Type Problems|9 VideosCABOXYLIC ACIDS AND ITS DERIVATIVES
HIMANSHU PANDEY|Exercise Subjective Type Problems|8 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-BIOMOLECULES-Level 2 (Q.51 To Q.55)
- A tripeptide is written as Glycine-Alamine-Glucine. The correct struct...
Text Solution
|
- What would be the net charge on the given amino acid at pH=14? H(2)O...
Text Solution
|
- How many moles of HIO(4) is required to break down the given molecule ...
Text Solution
|
- The prouduct of HIO(4) oxidation of the following compounds is :
Text Solution
|
- Which of the following compounds is D-aldopentose?
Text Solution
|