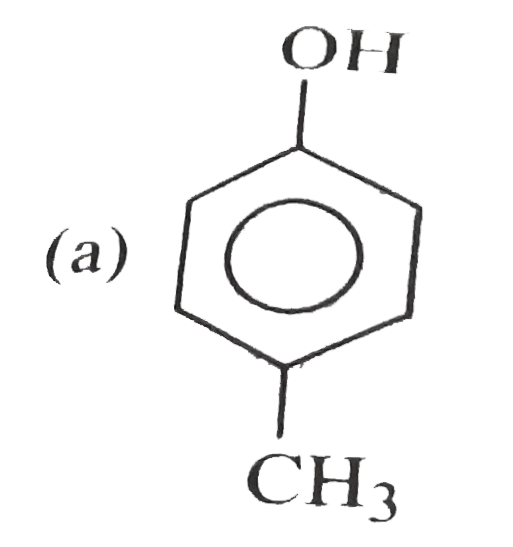
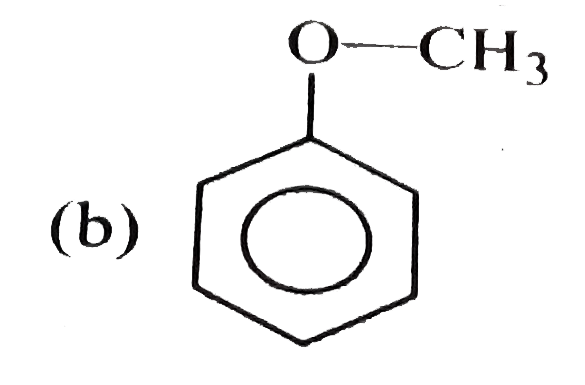
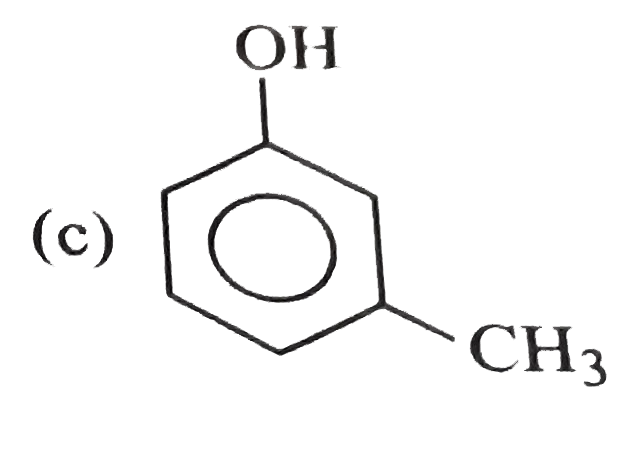
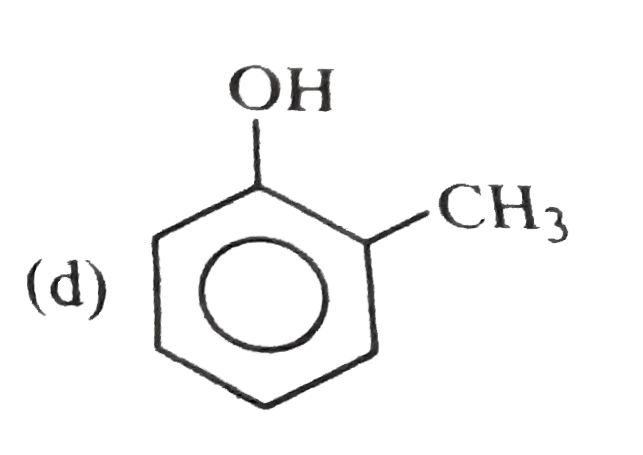
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-PRACTICAL ORGANIC CHEMISTRY-Match The Column
- Compound 'P', C(7)H(8)O is insolution in water , dilute when HCI and N...
Text Solution
|
- {:("Column" (I) , "Column" (II)) , ((a) Ph-overset(O)overset(||)(C)-CH...
Text Solution
|
Text Solution
|
- Match the following columns
Text Solution
|
Text Solution
|
- Match the following columns
Text Solution
|
- {:("Column" (I) , "Column"(II)), ((a) "Presence of halogen" , P. HNO(3...
Text Solution
|