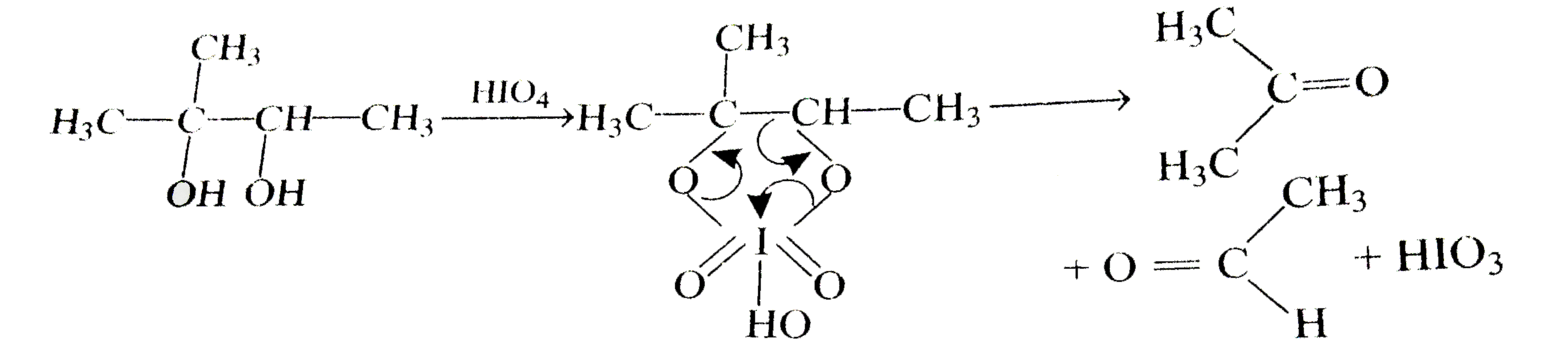
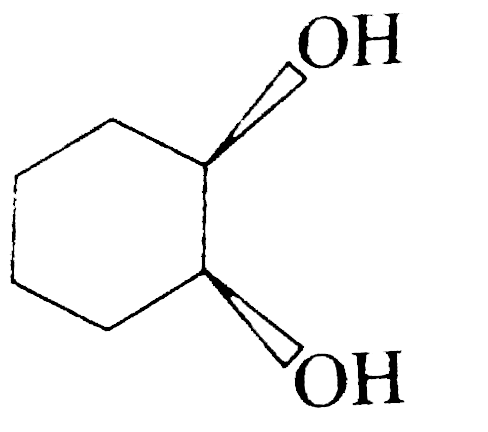
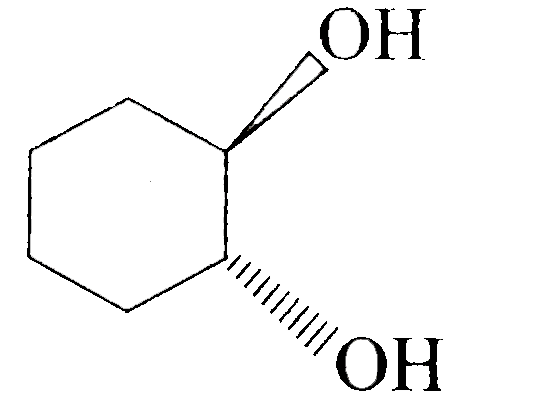
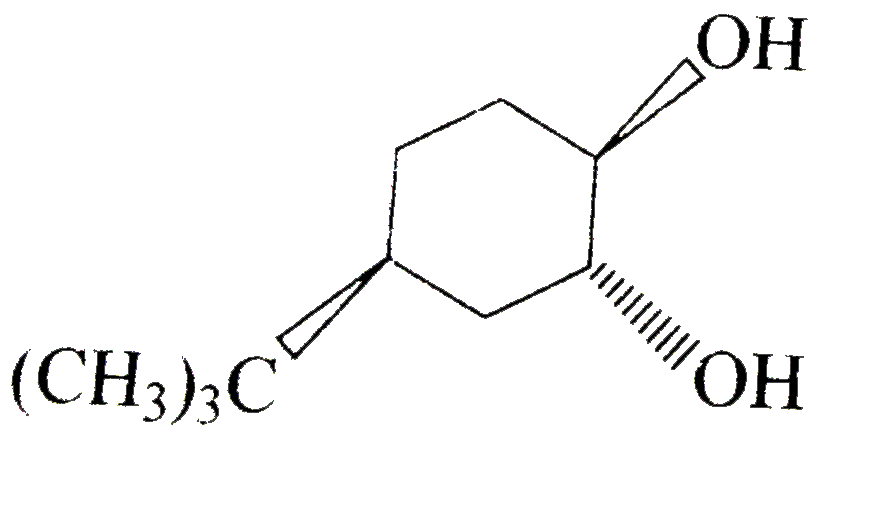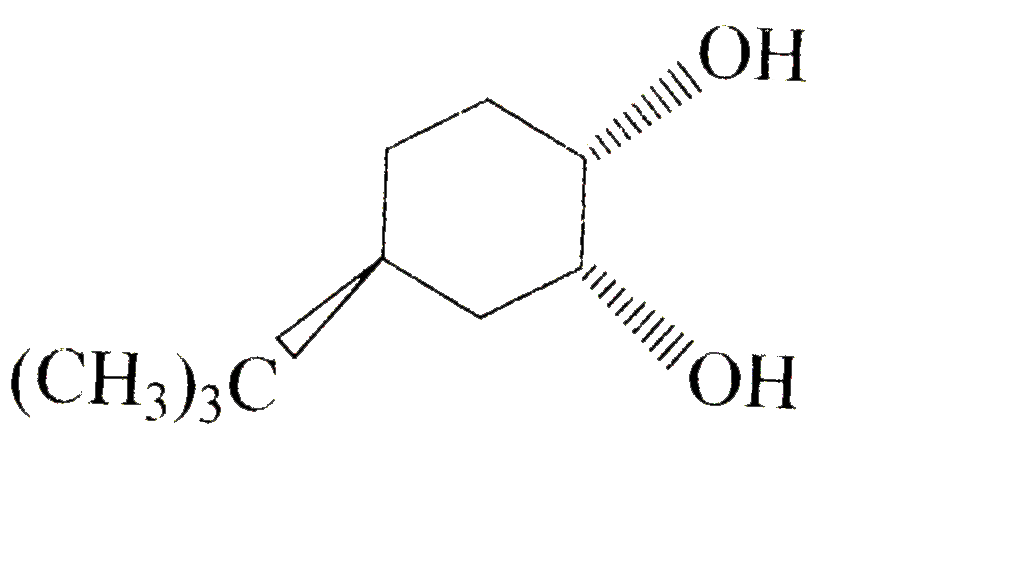A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1,2-diols are oxidised to ketones or aldehydes by periodic acid HIO(4)...
Text Solution
|
- Which of the following compound are oxidised by HIO(4)?
Text Solution
|
- Which compound will be oxidised by HIO(4) ?
Text Solution
|
- 1,2-diols are oxidised to ketones or aldehydes by periodic acid HIO(4)...
Text Solution
|
- 1,2-diols are oxidised to ketones or aldehydes by periodic acid HIO(4)...
Text Solution
|
- 1,2-diols are oxidised to ketones or aldehydes by periodic acid HIO(4)...
Text Solution
|
- What are the order of rates of oxidation with HIO(4) of the following ...
Text Solution
|
- The compound formed by reaction of ethylene glycol an periodic acid (H...
Text Solution
|
- What is formed when glycerol reacts with HIO(4) ?
Text Solution
|