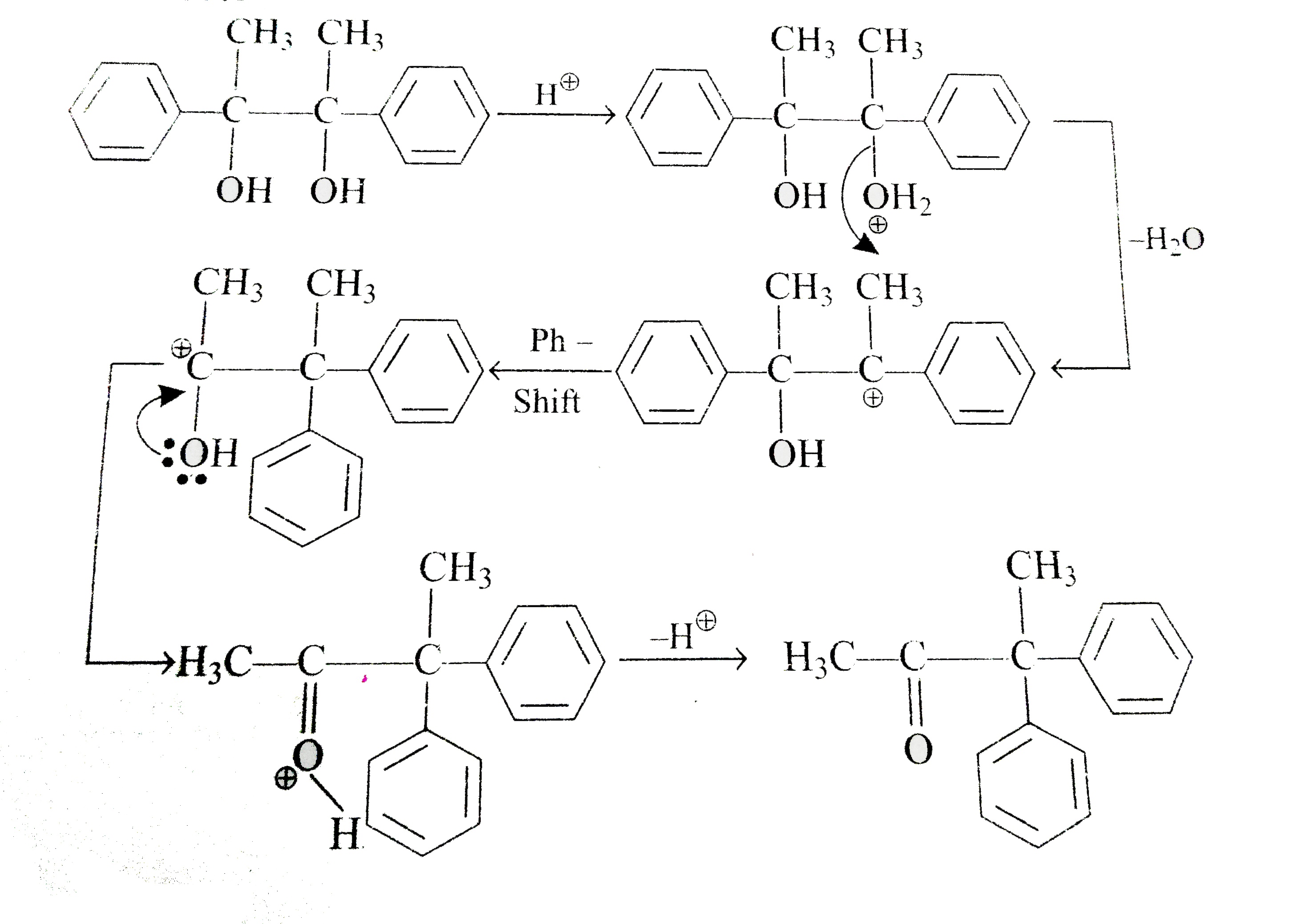
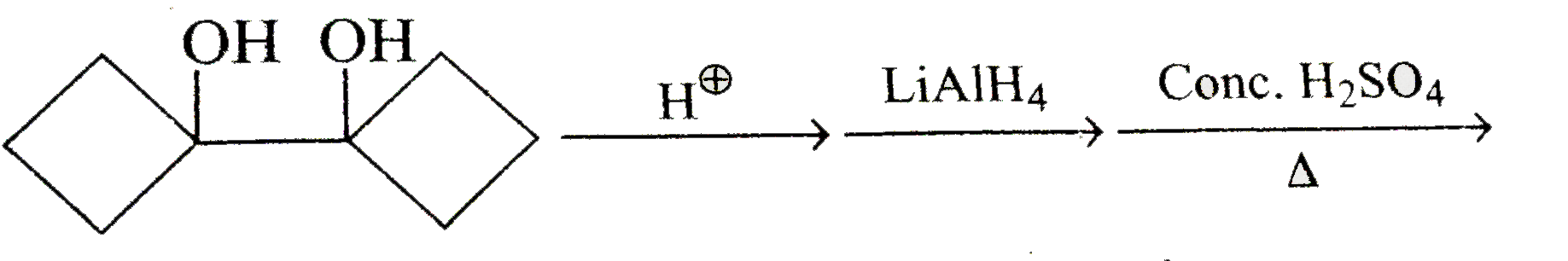
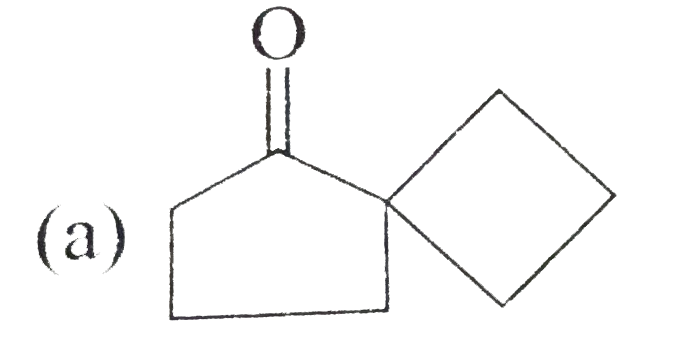
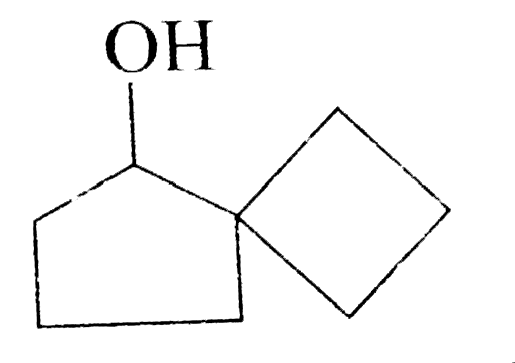
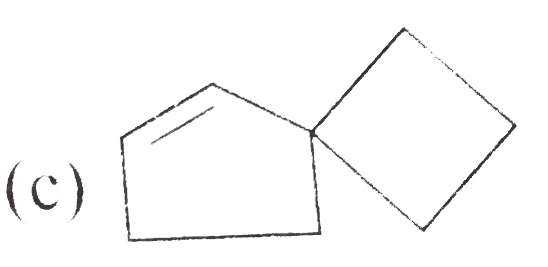
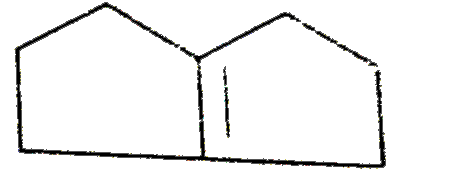
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Acid catalysed conversation of 1,2-diol or vicinal, into carbonyl comp...
Text Solution
|
- Acid catalysed conversation of 1,2-diol or vicinal, into carbonyl comp...
Text Solution
|
- The base catalysed reaction of 1,2-diketone to a salt of 2-hydroxy car...
Text Solution
|
- The base catalysed reaction of 1,2-diketone to a salt of 2-hydroxy car...
Text Solution
|
- Acid catalysed conversation of 1,2-diol or vicinal, into carbonyl comp...
Text Solution
|
- Acid catalysed conversation of 1,2-diol or vicinal, into carbonyl comp...
Text Solution
|
- Acid catalysed conversation of 1,2-diol or vicinal, into carbonyl comp...
Text Solution
|
- The less stable carbocation rearranges to more stable carbocation ion....
Text Solution
|
- (A) Give the mechanism of pinacol-pinacolone rearrangement reaction. (...
Text Solution
|