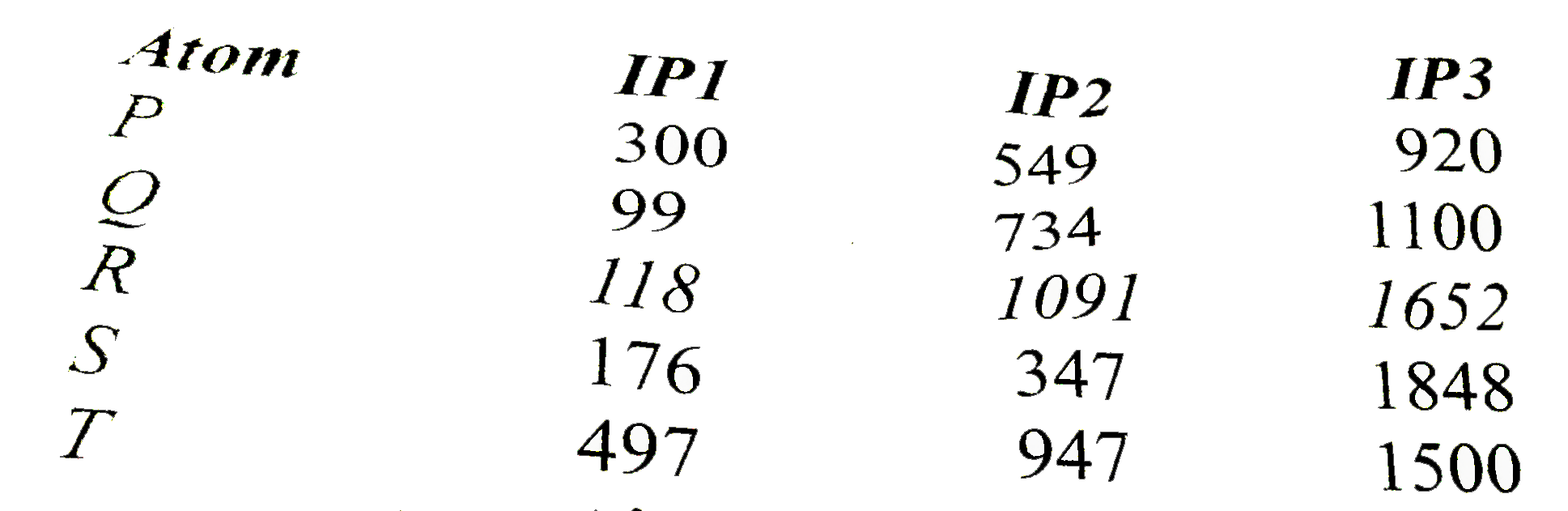
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-PERIODIC TABLE -All Questions
- Match the atomic numbers of the elements given in column I with the pe...
Text Solution
|
- Match the list-I and List-II and select the correct answer using the c...
Text Solution
|
- Ionization energies of five elements of same period in kcal/mol are gi...
Text Solution
|
- Ionization energies of five elements of same period in kcal/mol are gi...
Text Solution
|
- Ionization energies of five elements of same period in kcal/mol are gi...
Text Solution
|
- Ionization energies of five elements of same period in kcal/mol are gi...
Text Solution
|
- Which of the following is not an actinoid?
Text Solution
|
- The electronic configuration of gadolinium (Atomic number 64) is
Text Solution
|
- The statement that is not correct for periodic classification of eleme...
Text Solution
|
- The period number in the long form of the periodic table is equal to
Text Solution
|
- The elements in which electrons are progressively filled in 4f-orbital...
Text Solution
|
- The formation of oxide ion O^(2-)(g) from oxygen atom requires first a...
Text Solution
|
- Electronic configuration of four elements p,q,r and s are given below ...
Text Solution
|
- All transition elements are d-block elements, but all d-block elements...
Text Solution
|
- Identify the group nad valency of the elements having atomic number 11...
Text Solution
|
- Among the elements B,Al,C and Si a. Which has highest IE(1) ? b. W...
Text Solution
|
- Match the correct atomic radius with the element, in the increasing or...
Text Solution
|
- Match the correct ionisation enthalpies and electron gain enthalpies o...
Text Solution
|
- Electronic configuration of some elements is given in Column I and the...
Text Solution
|
- Which of the following elements can show covalency greater than 4?
Text Solution
|