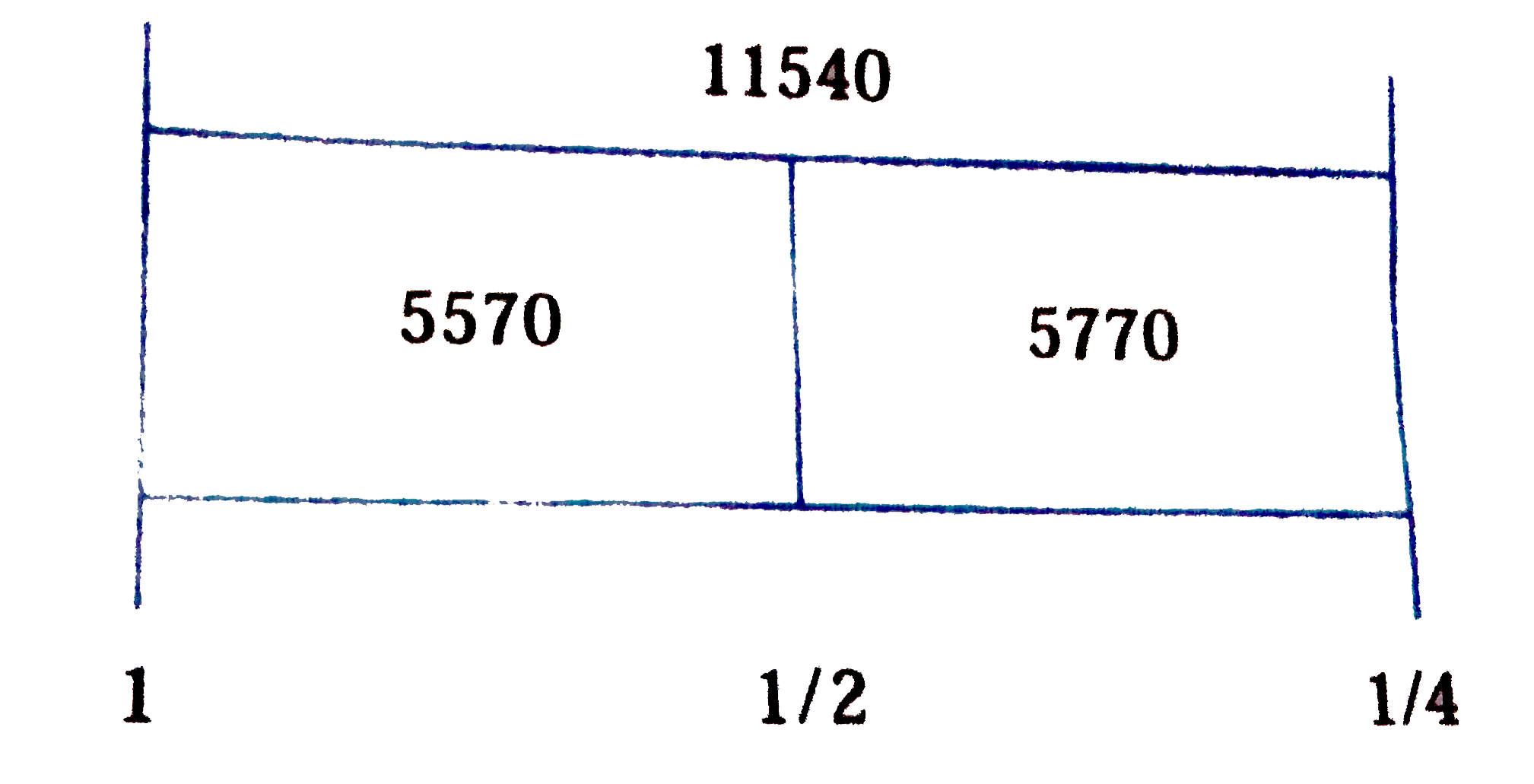
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-NUCLEAR CHEMISTRY-INTEGER
- Radioactive decay is a first- order process. Radioactive carbon in woo...
Text Solution
|
- Calculate difference between number of a beta particles emmited in the...
Text Solution
|
- How many alpha-particles will be emmited when ""(90)Th""^(234) change ...
Text Solution
|
- After 20 min, the amount of certai radioactive substance disintegrate ...
Text Solution
|
- At 27""^(@)C 100mL of 0.1 M radium chloride is 10""^(-3) Curies active...
Text Solution
|
- Iiodine -131 is a radioactive isdotpe. If 1.0 mg of ""^(131)I has an a...
Text Solution
|
- The average life of a radioactive element is 7.2 min. Calculate the ti...
Text Solution
|
- A,B and C are isodiaphers while C,D and E are isobers. Calculate the d...
Text Solution
|
- A radioactivity sample had an initial activity of 56dpm. After 69.3 mi...
Text Solution
|
- Na""^(22) has half life of 2.68 years. It decays both by positron emis...
Text Solution
|
- In how many ways among the following given, a nuicleus with high n/p r...
Text Solution
|
- A mixture of Pu""^(239) and Pu""^(240) has specific activity of 6xx10"...
Text Solution
|
- How many types of particles among the following are emitted during the...
Text Solution
|
- Assuming the age of the earth to be 10""^(10) yaers, the perccentage o...
Text Solution
|
- The periosic table consists of 18 groups. An isotope of copper, on bom...
Text Solution
|
- Assertion (A) : Nucleus of the atom does not contain electrons,yet it ...
Text Solution
|
- Assertion (A) : gamma-rays have very high penetrating power. Reason ...
Text Solution
|
- Assertion (A) : beta-particles have greater penetrating power than alp...
Text Solution
|
- Assertion (A) : The average life of a radioactive element is infinity ...
Text Solution
|
- Assertion (A) : Half life of a radioactive isotope is the time require...
Text Solution
|
- Assertion (A) : K-shell electron capture is detected by anylizing the...
Text Solution
|