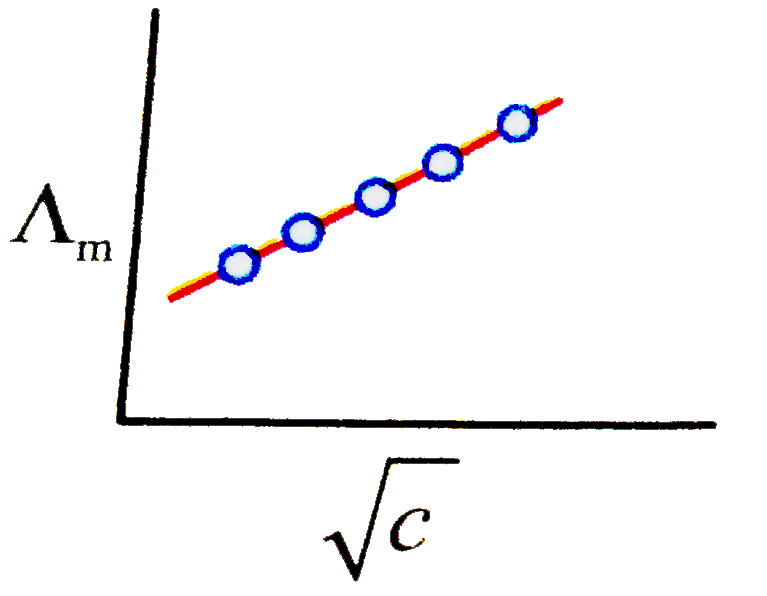
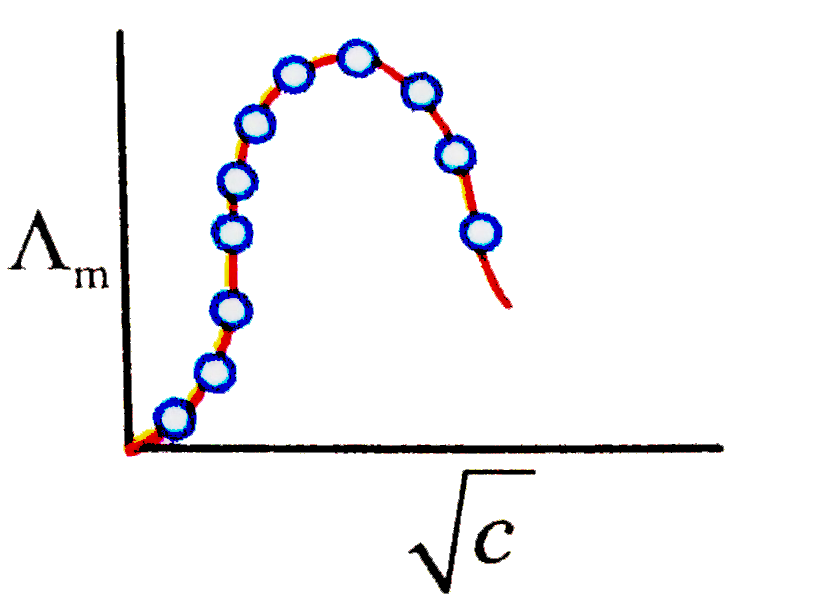
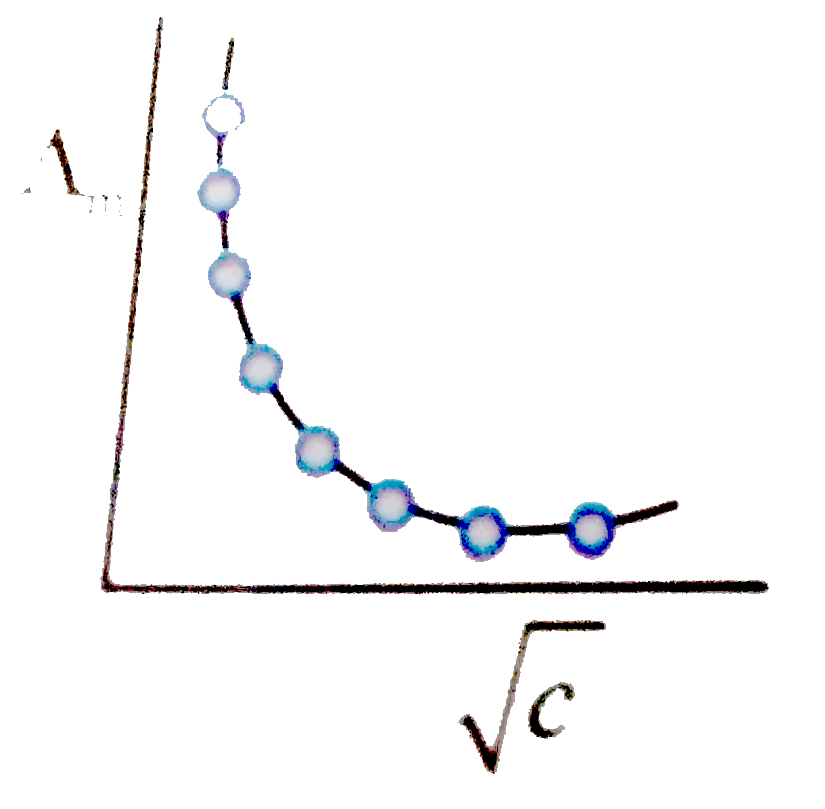
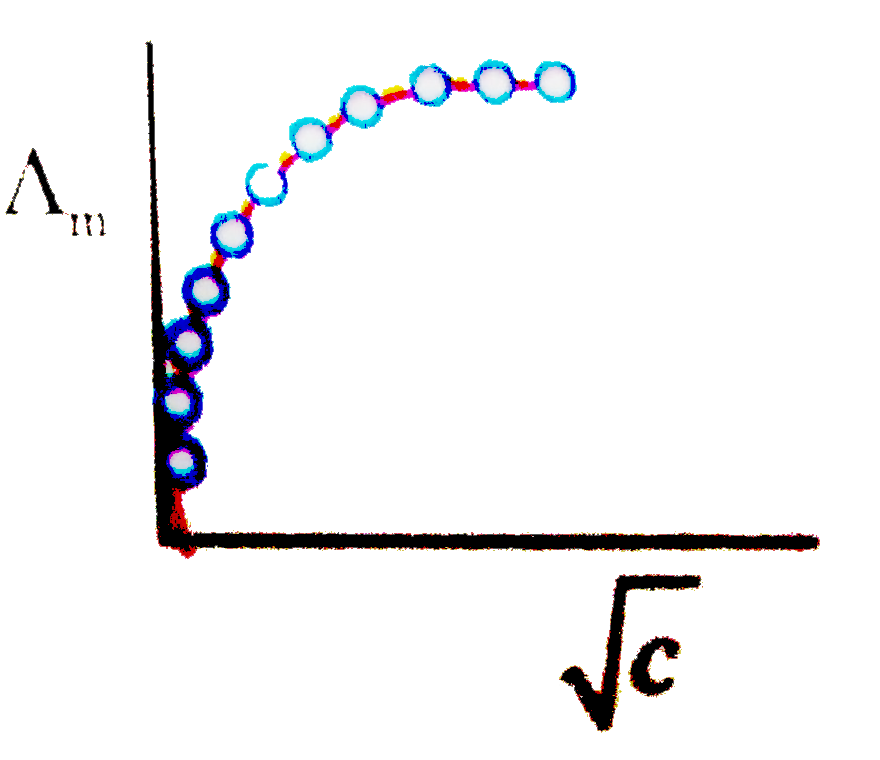
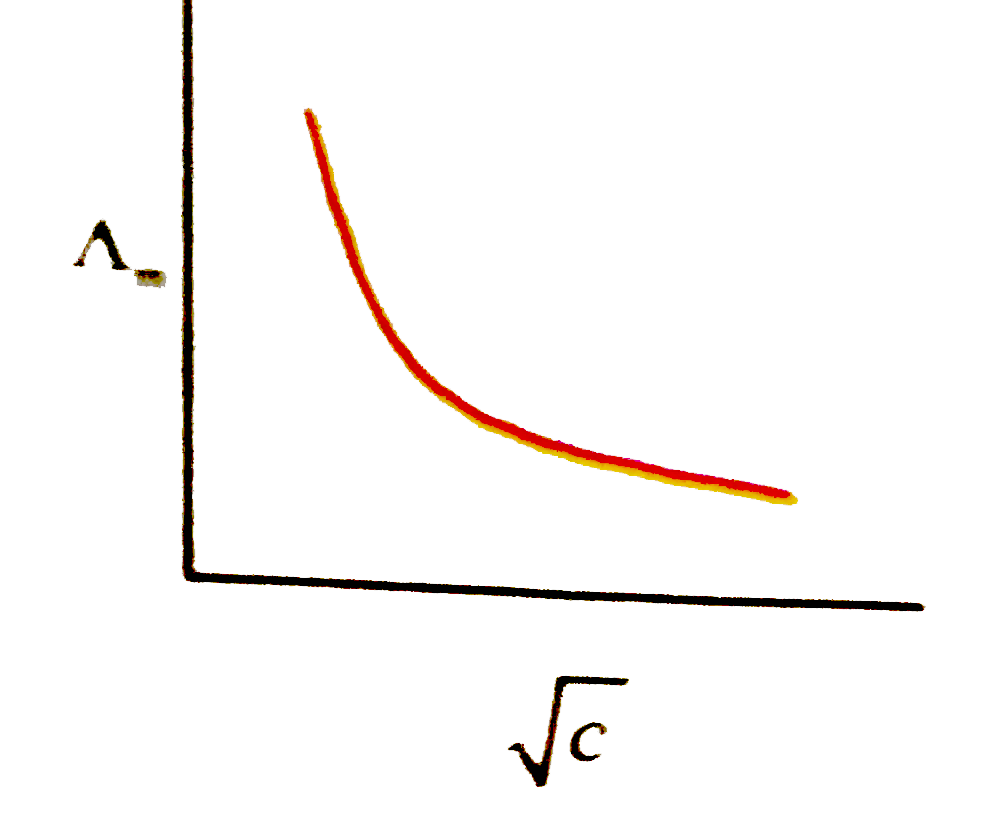A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA- ELECTRO CHEMISTRY-LEVEL-I(C.W)
- The correct order of equivalent conductance at infinite dilution of Li...
Text Solution
|
- Which of the following solution of KCl has the lowest value of specifi...
Text Solution
|
- The variation of ^^(m) of acetic acid with concentration is correctly ...
Text Solution
|
- The molar conductance of acetic acid at infinite dilution is lambda(oo...
Text Solution
|
- According of Kohlrausch law, the limiting value of molar conductivity ...
Text Solution
|
- Molar conductance of BaCl(2),H(2)SO(4) and HCl at infinite dilutions a...
Text Solution
|
- The SRP values of Ag^(+)//Ag and Zn^(2+)//Zn electrodes are 0.80V and ...
Text Solution
|
- Which of the following is most powerful oxidizing agent?
Text Solution
|
- The standard reduction potentials of Cu^(=2),Ag^(+),Hg^(+2) and Mg^(+2...
Text Solution
|
- Which metal pairs when coupled will get maximum emf for a voltaic cell
Text Solution
|
- The standard reduction potentials at 298K, for the following half cell...
Text Solution
|
- Cu-2e^(-)rarrCu^(2+),E^(@)=-0.347V Sn-2e^(-)rarrSn^(2+),E^(@)=+0.143...
Text Solution
|
- The voltage of a cell whose half-cells are given below is Mg^(2+)+2e...
Text Solution
|
- The standard reduction potentials of Ag, Cu, Co and Zn are 0.799,0.337...
Text Solution
|
- When zinc is added to CuSO(4) solution, copper is precipitated. It is ...
Text Solution
|
- Consider the following four electrodes: A=Cu^(2+)(0.0001M)//Cu((s)) ...
Text Solution
|
- Which of the following is always true regarding the spontaneity of rea...
Text Solution
|
- The reduction potential of hudrogen electrode is- 118 mV. The concentr...
Text Solution
|
- E^(@) for F(2)+2e^(-) hArr 2F^(-) is 2.8V,E^(@) for (1)/(2)F+e^(-)=F^(...
Text Solution
|
- On electrolysing K(2)SO(4) solution using inert electrodes, 1.68L(STP)...
Text Solution
|