Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA- ELECTRO CHEMISTRY-SUBJECTIVE QUESTIONS
- Two students use same stock solution of ZnSO(4) and a solution of CuSO...
Text Solution
|
- Find the equilibrium constant that the reaction In^(2+)+Cu^(2+)under...
Text Solution
|
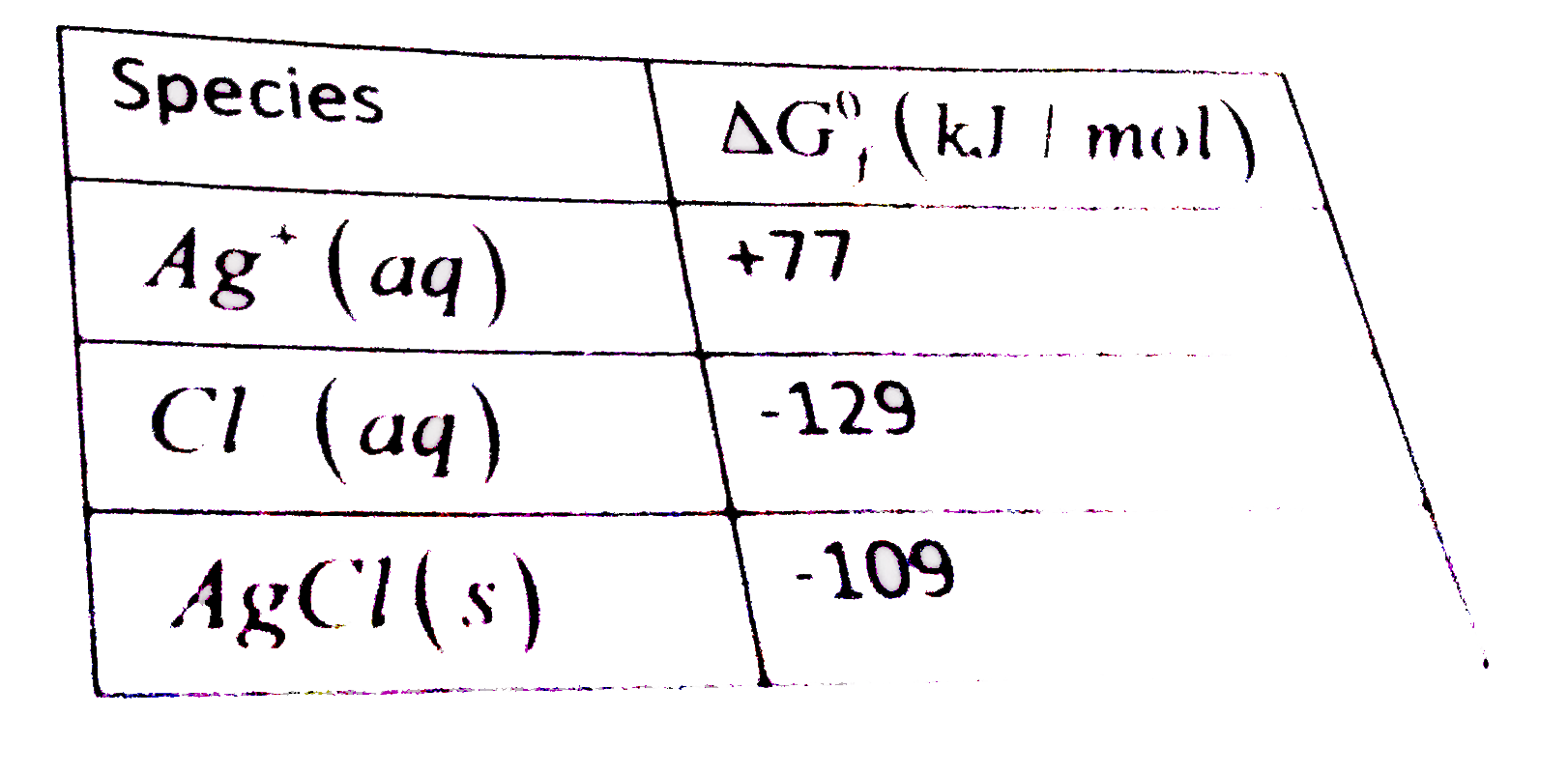
- a. For the reaction Given : Ag^(o+)(aq)+Cl^(o+)(aq)rarr AgCl(s) ...
Text Solution
|
- We have taken a saturated solution of AgBr,K(sp) of AgBr is 12xx10^(-1...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Tollen reagent is used for the detection of aldehydes. When a solution...
Text Solution
|
- Redox reactions play a pivotal role in chemistry and biology. The valu...
Text Solution
|
- Redox reactions play a pivotal role in chemistry and biology. The valu...
Text Solution
|
- Redox reactions play a pivotal role in chemistry and biology. The valu...
Text Solution
|
- The concentration of potassium ions inside a biological cell is at lea...
Text Solution
|
- The concentration of potassium ions inside a biological cell is at lea...
Text Solution
|
- The electrochemical cell shown below is a concentration cell M//M^(...
Text Solution
|
- The electrochemical cell shown below is a concentration cell. M|M^(2+)...
Text Solution
|
- Cd amalgam is prepared by electrolysis of a solution of CdCl(2) using ...
Text Solution
|
- The standard oxidation potential of Ni//Ni^(2+) electrode is 0.236V. I...
Text Solution
|
- Molar conductance of saturated BaSO(4) solution is 400 ohm^(-1)cm^(2)m...
Text Solution
|
- At equimolar concentration of Fe^(+2)//Fe^(+3), what must [Ag^(+)] be ...
Text Solution
|
- 3 ampere current was passed through an aqueous solution of an unknown ...
Text Solution
|
- An aqeous solution of X is added slowly to an aqueous solution of Y as...
Text Solution
|