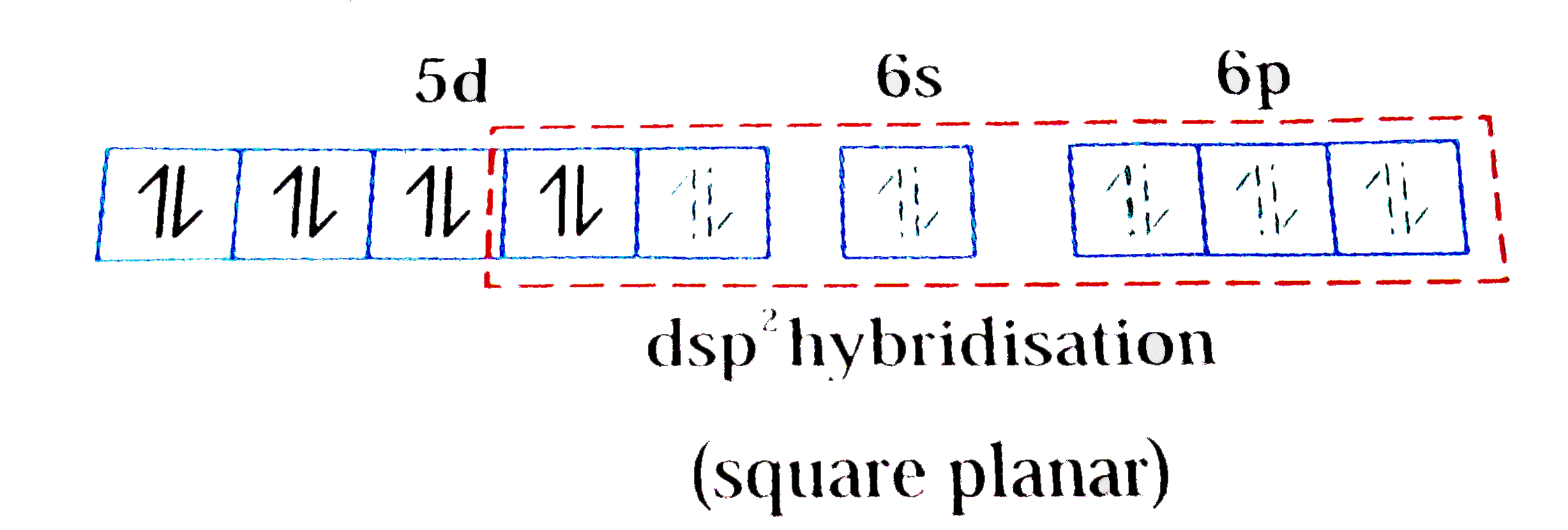
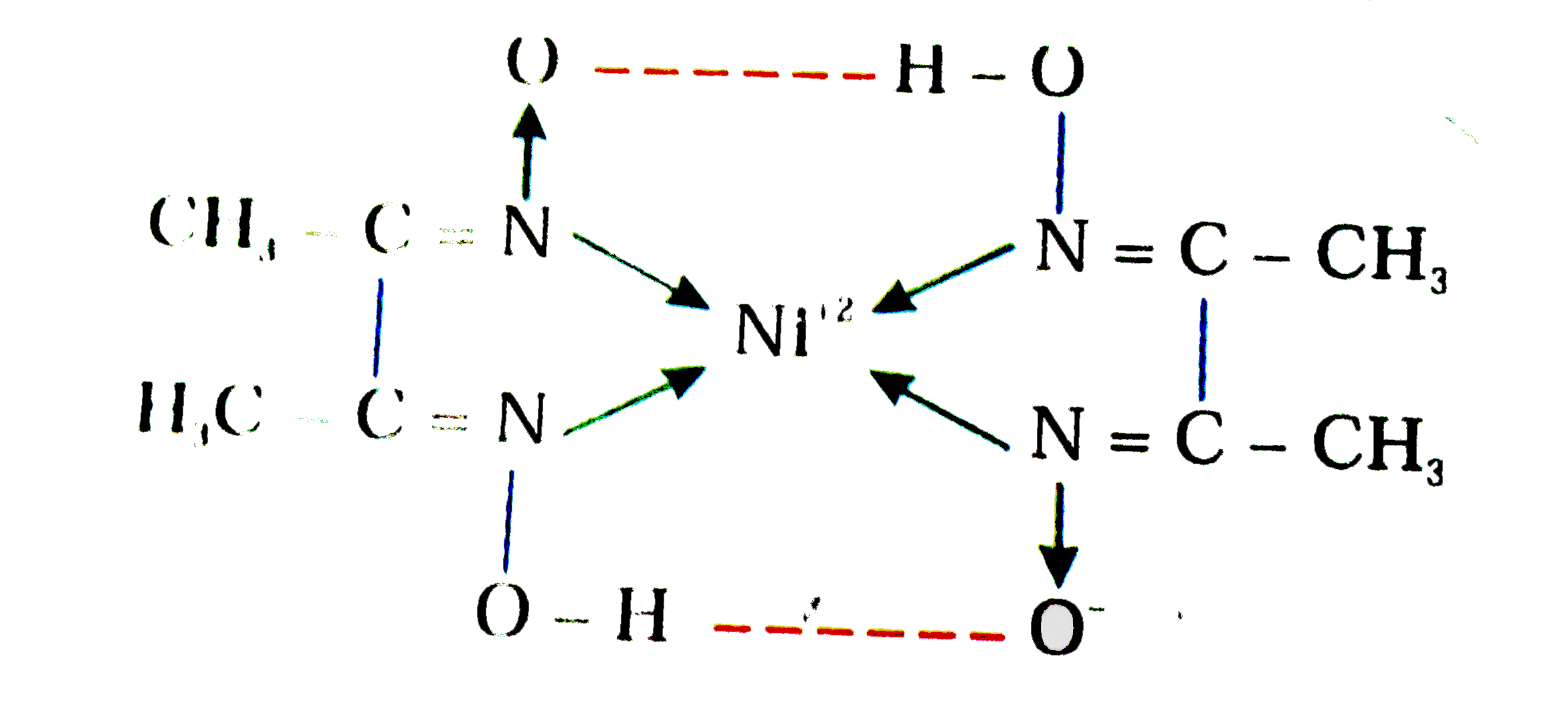
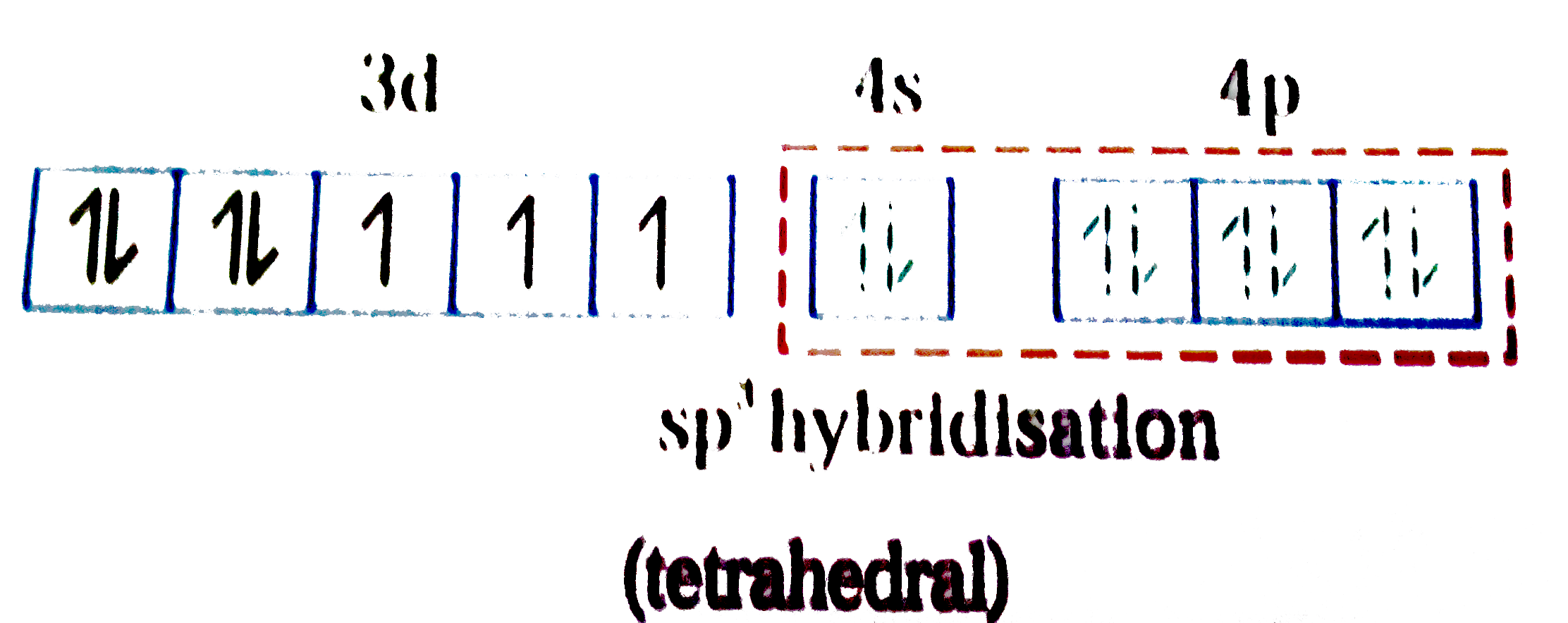A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPLEXES
NARAYNA|Exercise Comprehension-4|2 VideosCOORDINATION COMPLEXES
NARAYNA|Exercise Comprehension-5|2 VideosCOORDINATION COMPLEXES
NARAYNA|Exercise Comprehension-2|3 VideosCO-ORDINATE COMPOUNDS
NARAYNA|Exercise Exercise-4|60 VideosD - BLOCK ELEMENTS
NARAYNA|Exercise CHECK YOUR GRASP|7 Videos
Similar Questions
Explore conceptually related problems