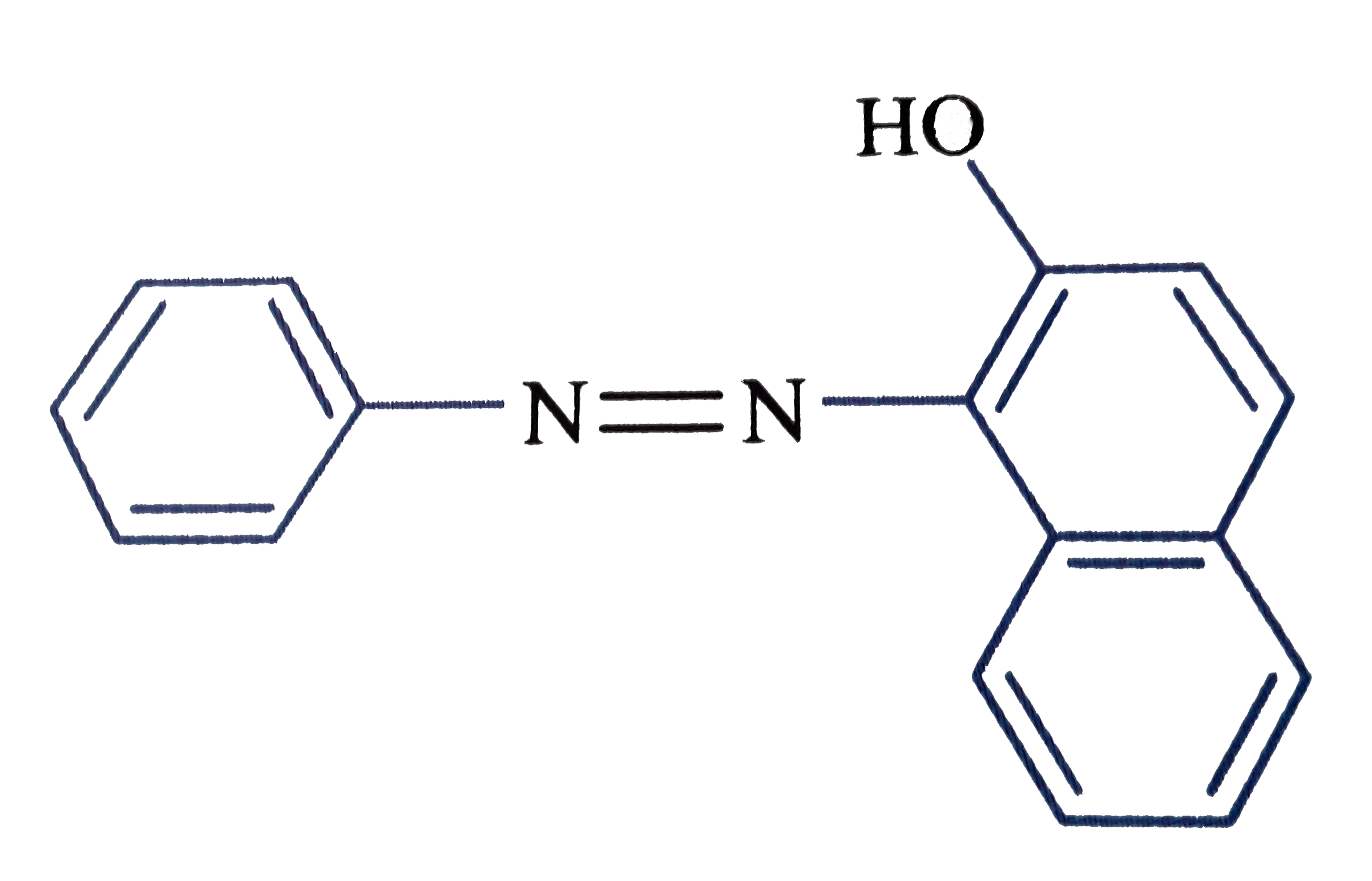
A
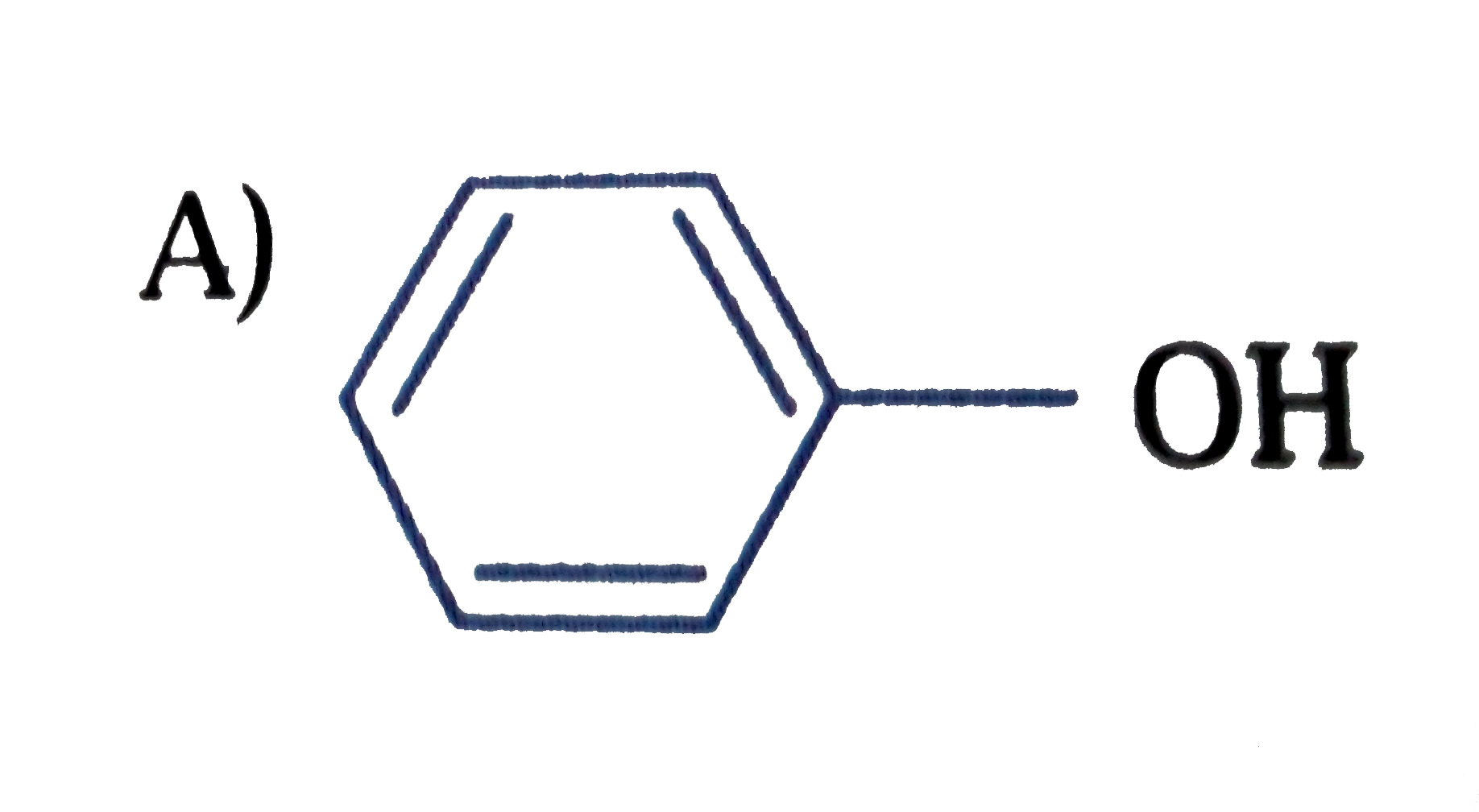
B
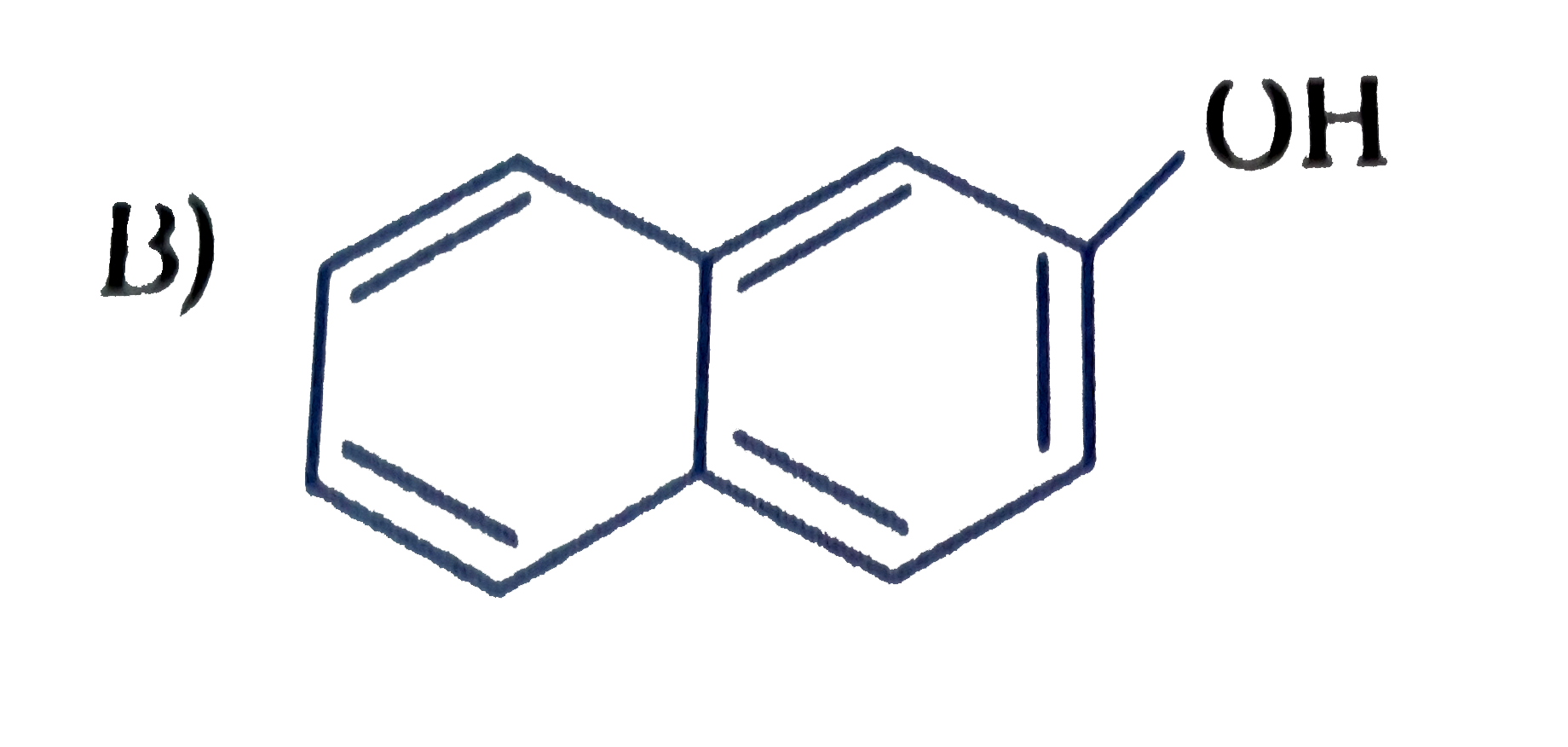
C
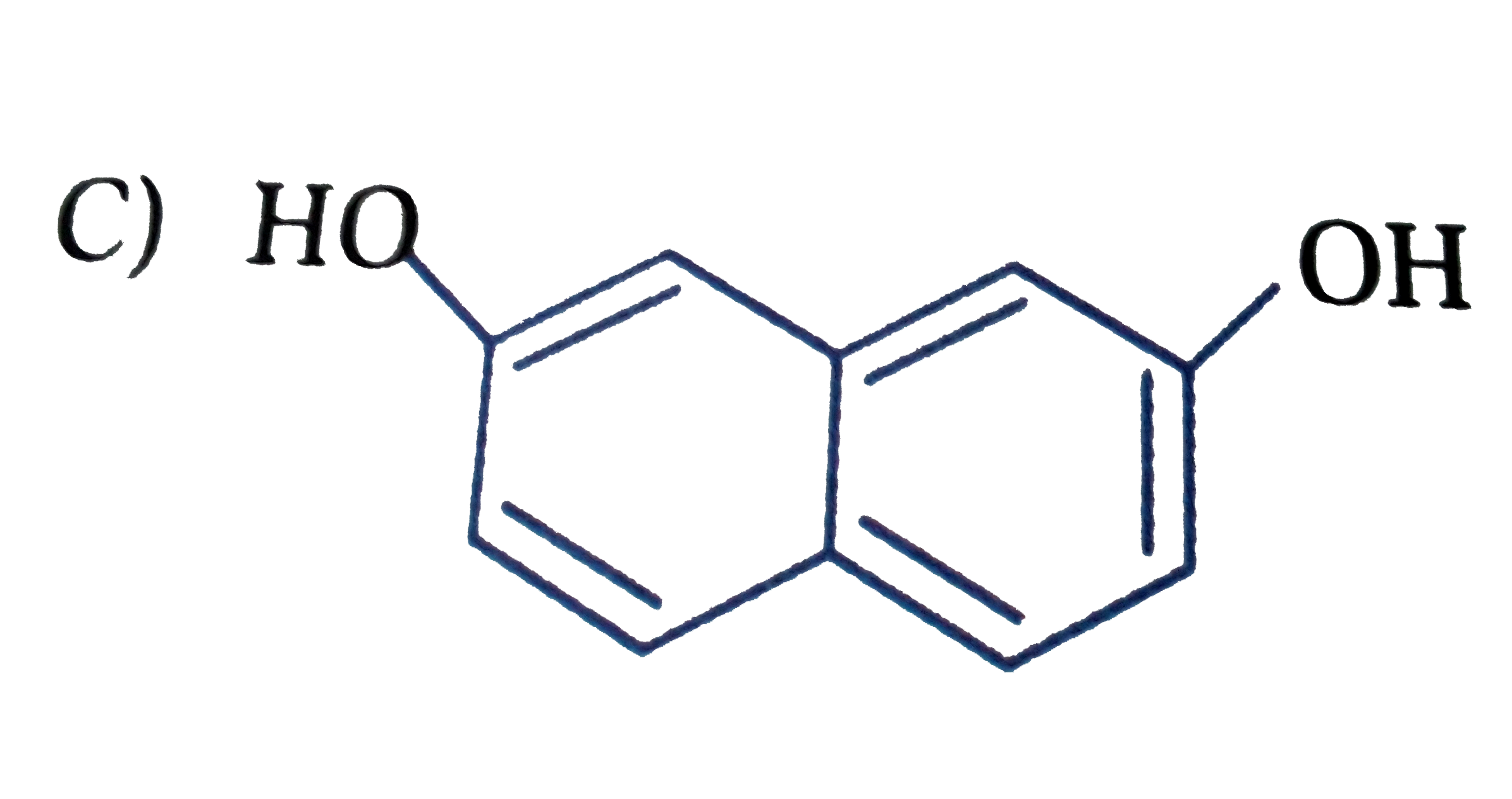
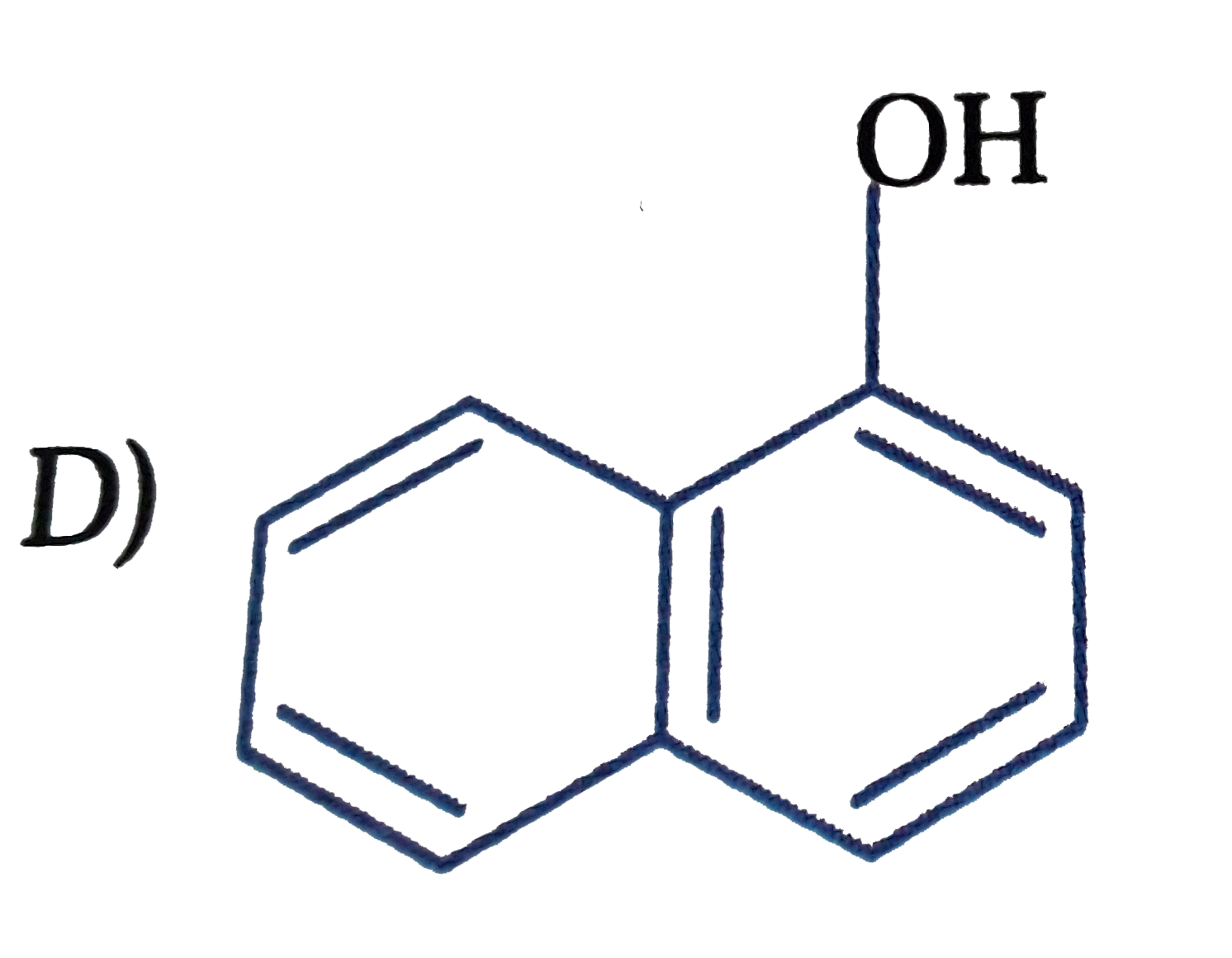
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AMINES & DIAZO COMPOUNDS
NARAYNA|Exercise Level -VI|12 VideosAMINES & DIAZO COMPOUNDS
NARAYNA|Exercise Multiple answre type q.|17 VideosAMINES & DIAZO COMPOUNDS
NARAYNA|Exercise Level II (H.W.)|15 VideosALDEHYDES, KETONES & CARBOXYLIC ACIDS
NARAYNA|Exercise CHECK YOUR GRASP|6 VideosBIOMOLECULES
NARAYNA|Exercise EXERCISE-4 (NCERT EXAMPLAR PROBLEMS)|12 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-AMINES & DIAZO COMPOUNDS-Level V
- is:
Text Solution
|
- Complete the following reaction
Text Solution
|
- The which is a red azo dye obtained on reacting benzene diazonium...
Text Solution
|
- The best method for the preparation of primary amines is
Text Solution
|
- Acetophenone can be converted into amine in a single step by
Text Solution
|
- .The final product is
Text Solution
|
- N-ethyl formamide on dehydration with POCl(3) in presence of pyridine ...
Text Solution
|
- Piperidine is a secondary amine, which is subjected to Hofmann elimina...
Text Solution
|
- An organic compound with molecular formula C3 H5 N on hydrolysis gives...
Text Solution
|
- R- C-=N can be reduced to RCH2 NH2 using the reducing agent.
Text Solution
|
- Tertiary amines may be obtained by:
Text Solution
|
- CH3 CH2 NH2 is soluble in
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|
- Which of the following amine(s) after diazotization will form deeply c...
Text Solution
|
- The amines that will give off N2 upon treatment with NaNO2 and dil. H...
Text Solution
|
- Reaction of R-overset(O)overset(||) C-NH2 with a mixture of Br2 and KO...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Assertion: Carbyl amine is given by secondary amines only Reason: An...
Text Solution
|