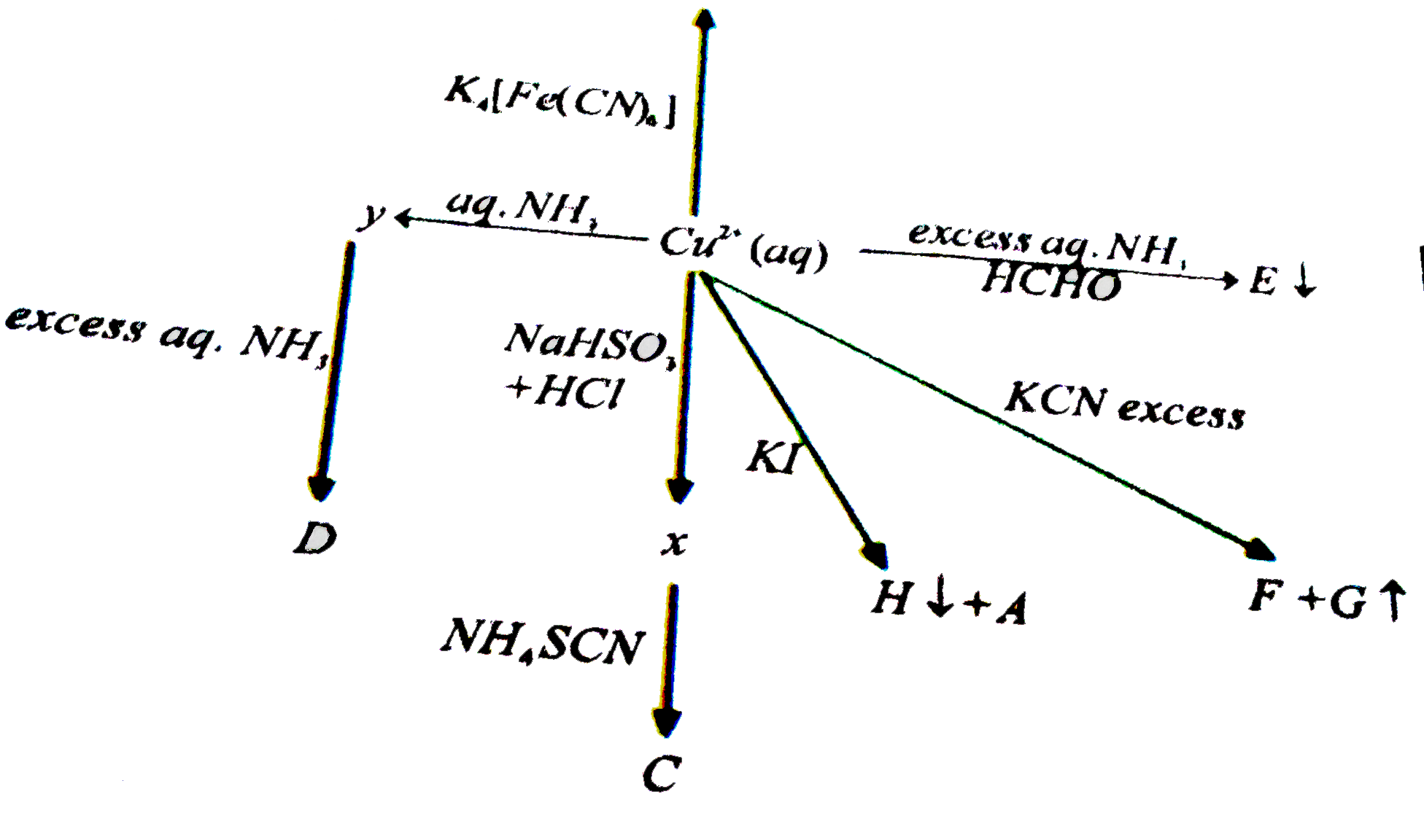
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMISTRY OF HEAVY METALS
NARAYNA|Exercise Passage 2|6 VideosCHEMISTRY OF HEAVY METALS
NARAYNA|Exercise passage 3|12 VideosCHEMISTRY OF HEAVY METALS
NARAYNA|Exercise Level VI|14 VideosCHEMISTRY IN EVERYDAY LIFE
NARAYNA|Exercise ASSERTION & REASON TYPE QUESTIONS|10 VideosCO-ORDINATE COMPOUNDS
NARAYNA|Exercise Exercise-4|60 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-CHEMISTRY OF HEAVY METALS-Passage 1
- The color of 'C' is
Text Solution
|
- The color of B is
Text Solution
|
- The geometry of the complex 'D' in the solid state is
Text Solution
|
- The oxidation state of Cu in the 'E' is
Text Solution
|
- The coordination number and oxidation state of copper in 'F' is
Text Solution
|
- When 'I' is what is 'A'
Text Solution
|
- Copper is the most noble of the first row transition metals and occurs...
Text Solution
|