`V_(1)=24.63 "L " T_(1)=300 K , T_(2)=600 K`
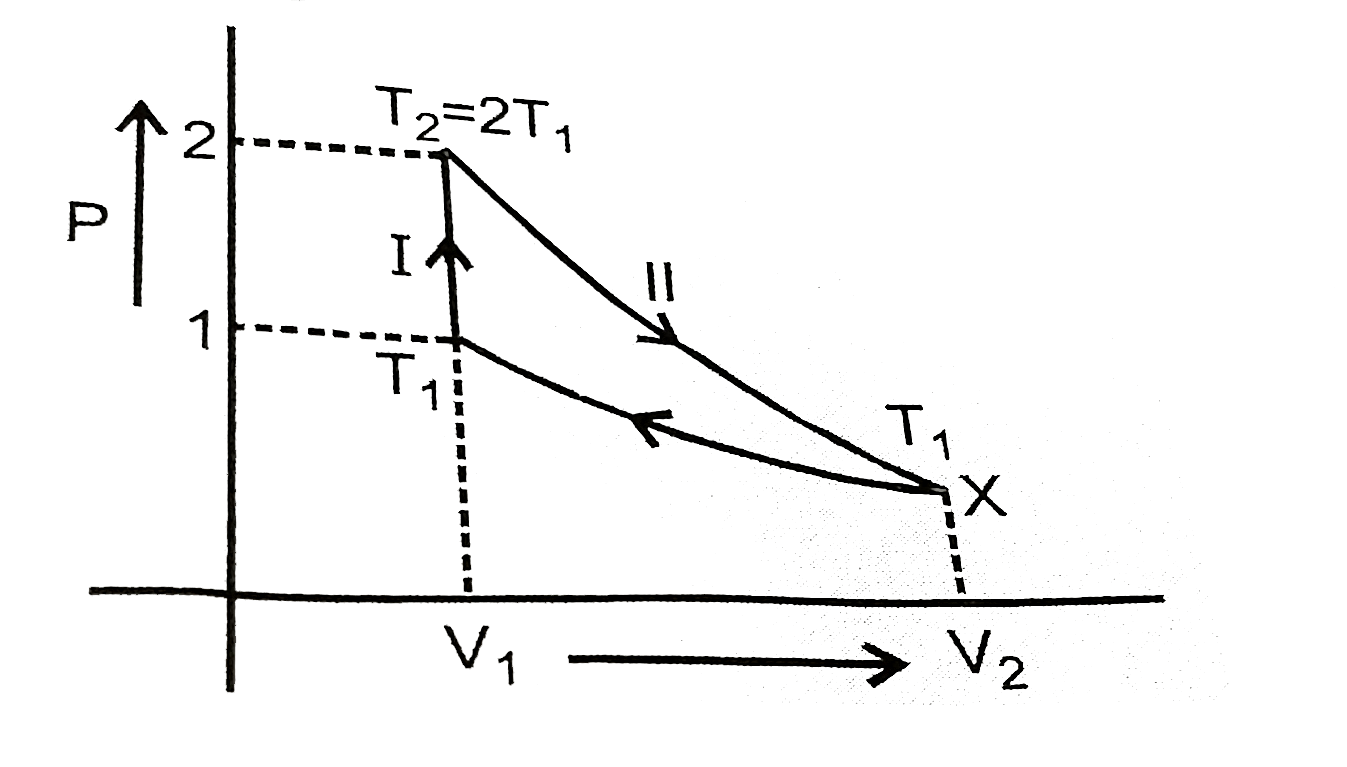
For path (II)
`T_(1)V_(1)^(gamma-1)=T_(2)V_(2)^(gamma-1)`
`(T_(1))/(T_(2))=((V_(2))/(V_(1)))^(gamma-1)`
`(600)/(300)=((V_(2))/(V_(1)))^((7)/(5)-1)`
`(2)^(5//2)=(V_(2))/(V_(1))`
`V_(2)=4sqrt(2)xx24.63 = 139.3 L~~ 139 L`