Di-tert-glycols rearrange in the presence of acid to give `alpha`-trtiary ketones. The trivial name of the simplest glycol of this type is pinacol, and this type of reaction therefore is named pinacol rearrangement (in this specific case, the reaction is called a pinacol-pinacolone rearrangement). The rearrangement involves 4 steps. one of the hydroxyl group is protonated in the first step. A molecule of water is eliminated in the second step and a tertiary carbocation is formed. the carbocation rearranges in the third step into a more stable carboxonium ion via a [1, 2] rearrangement. In the last step, the carboxonium ion is deprotonated and the product ketone is obtained.
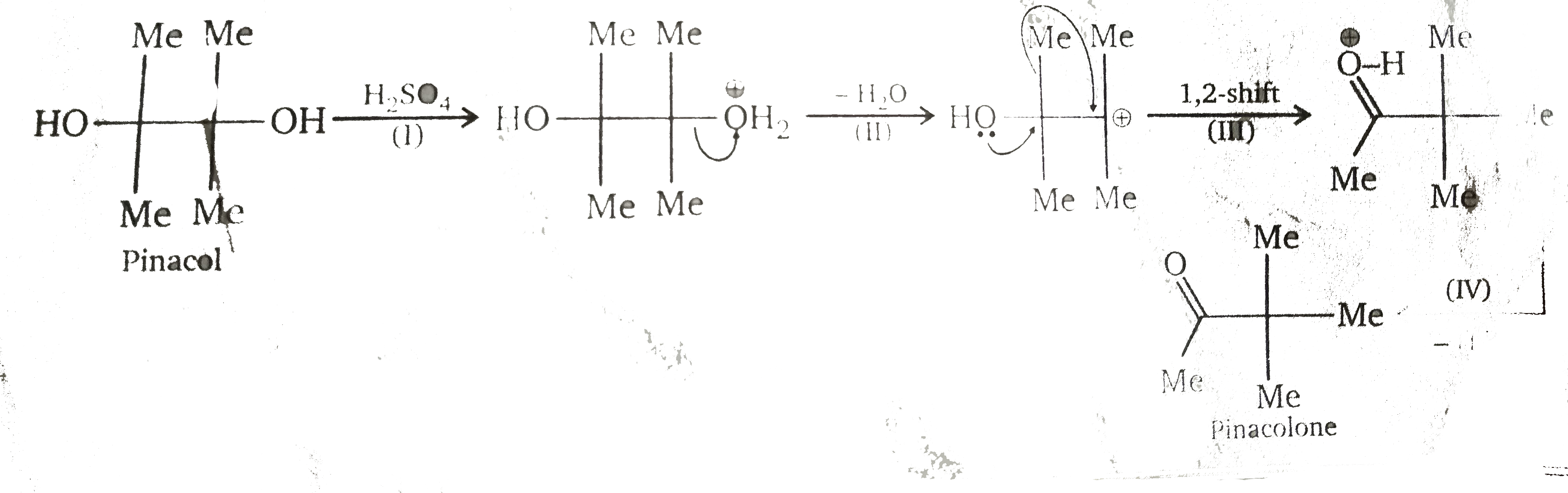
What is R.D.S. of pinacol-pinacolone rearrangement ?