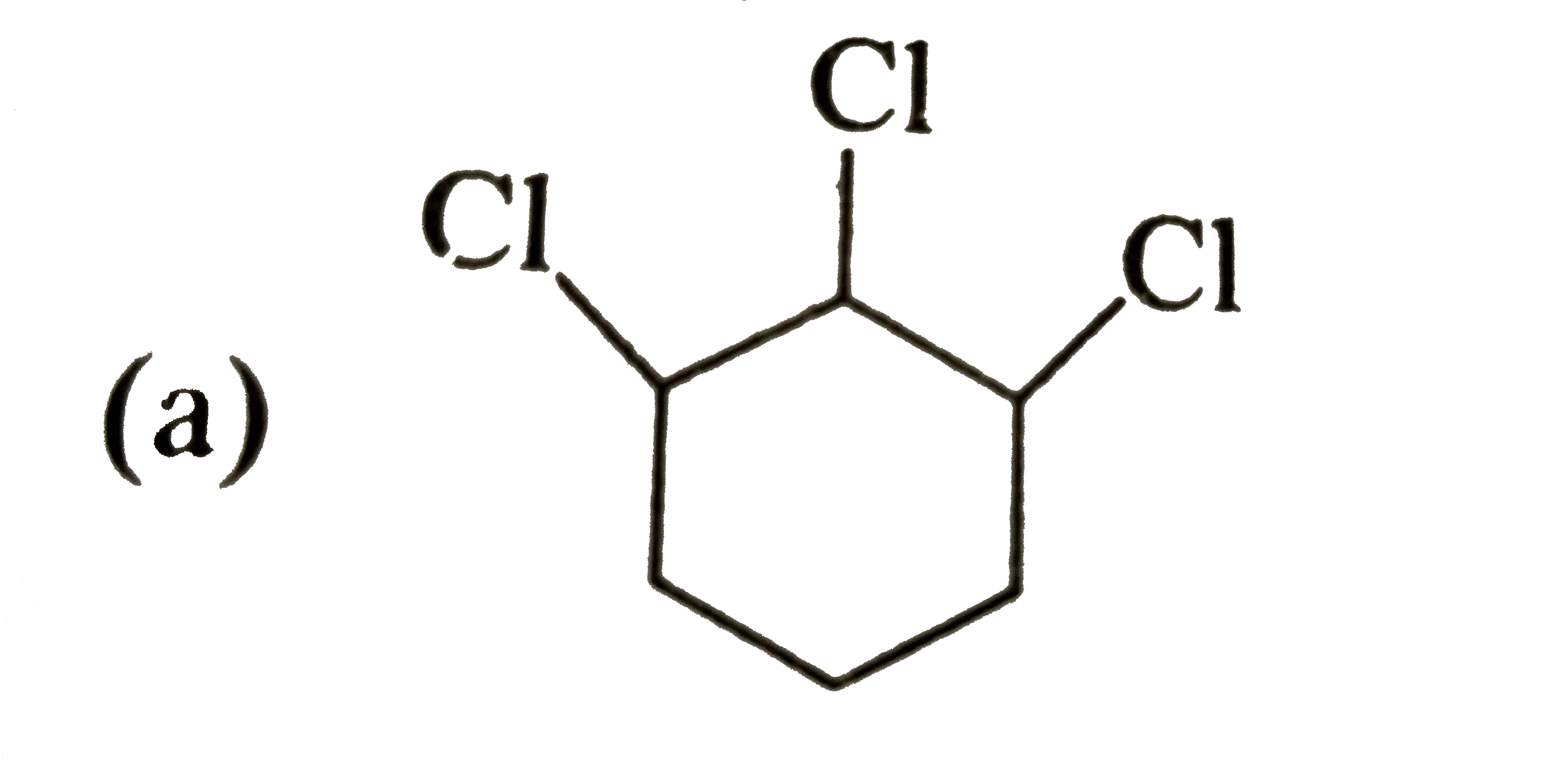
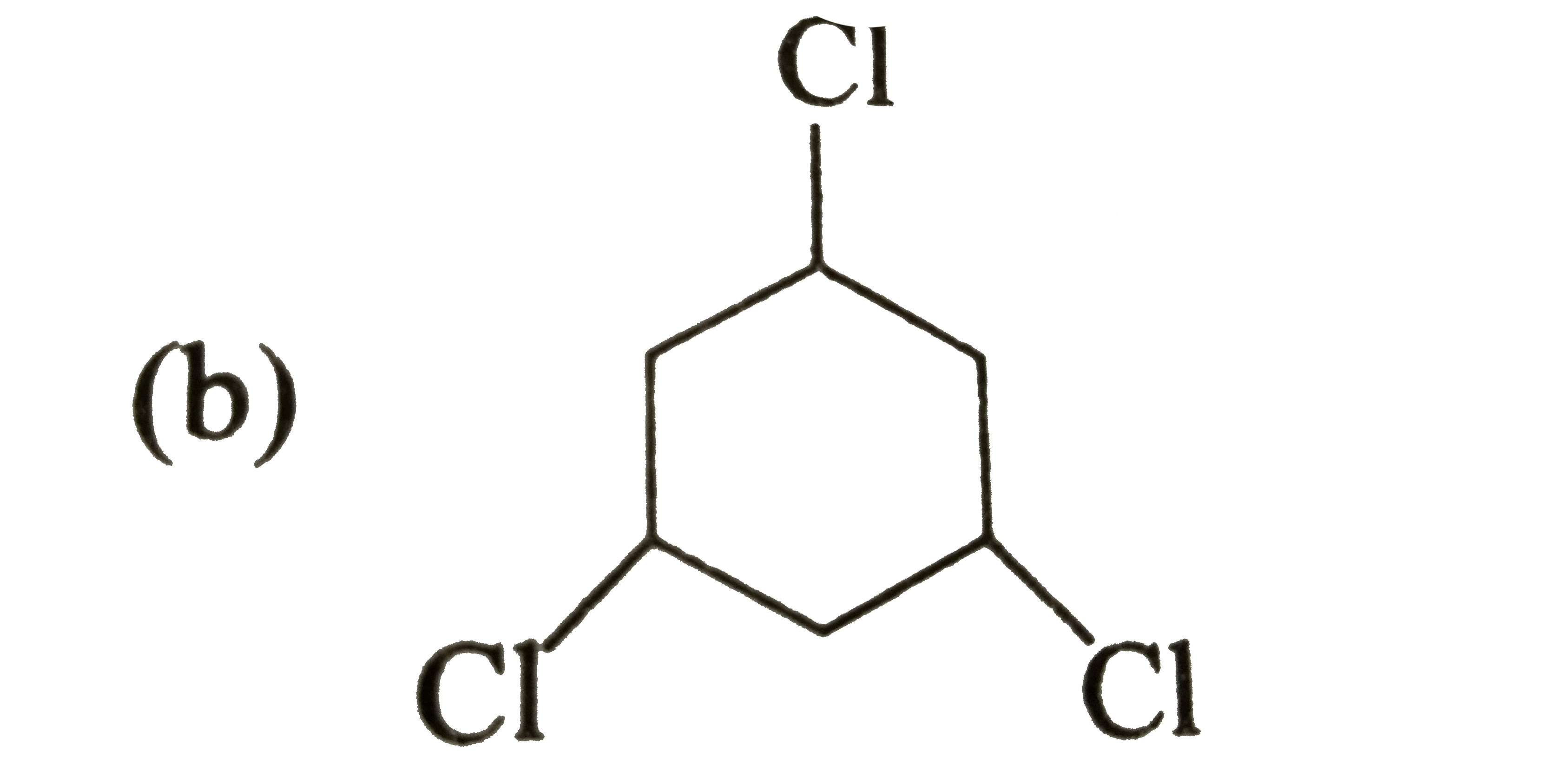
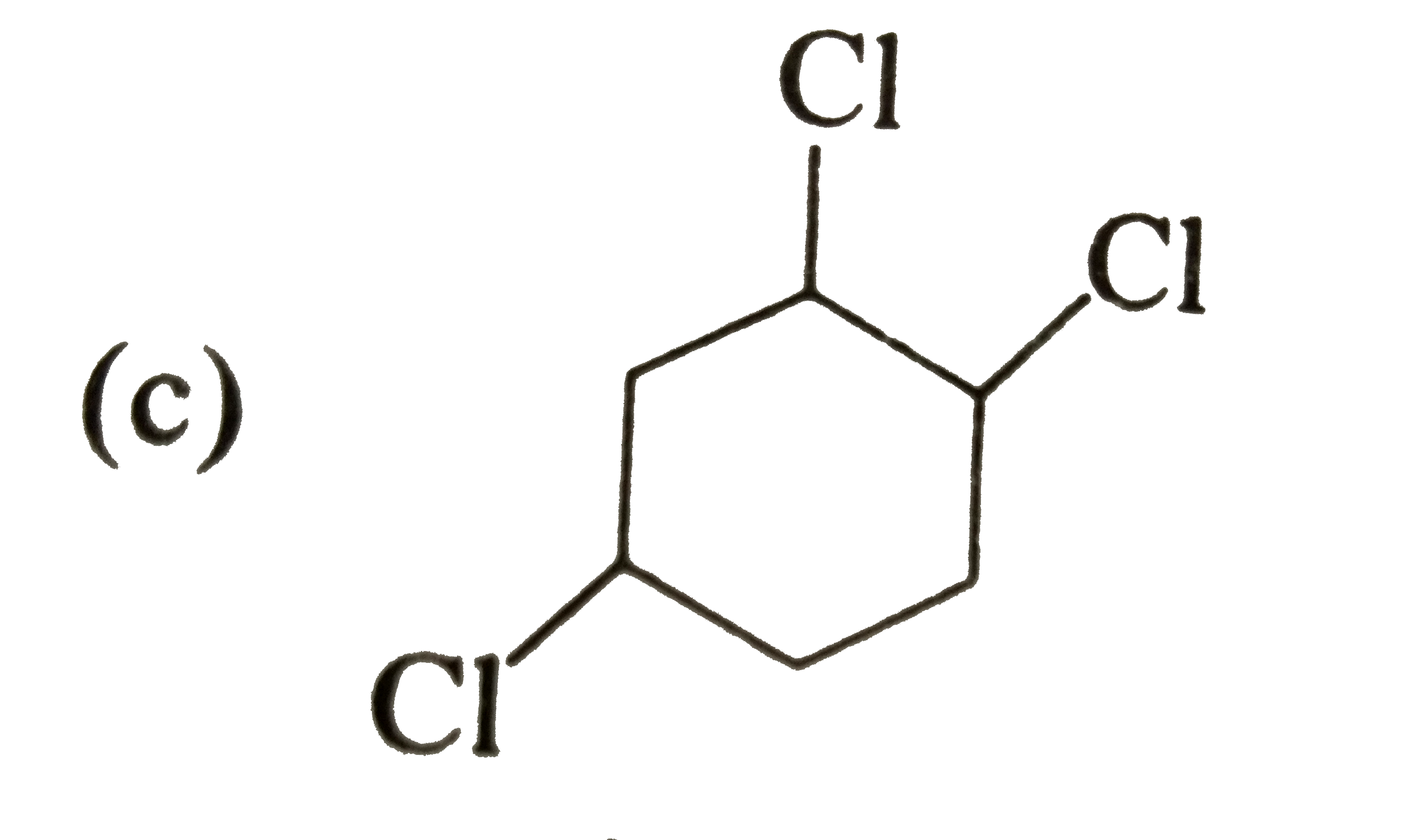
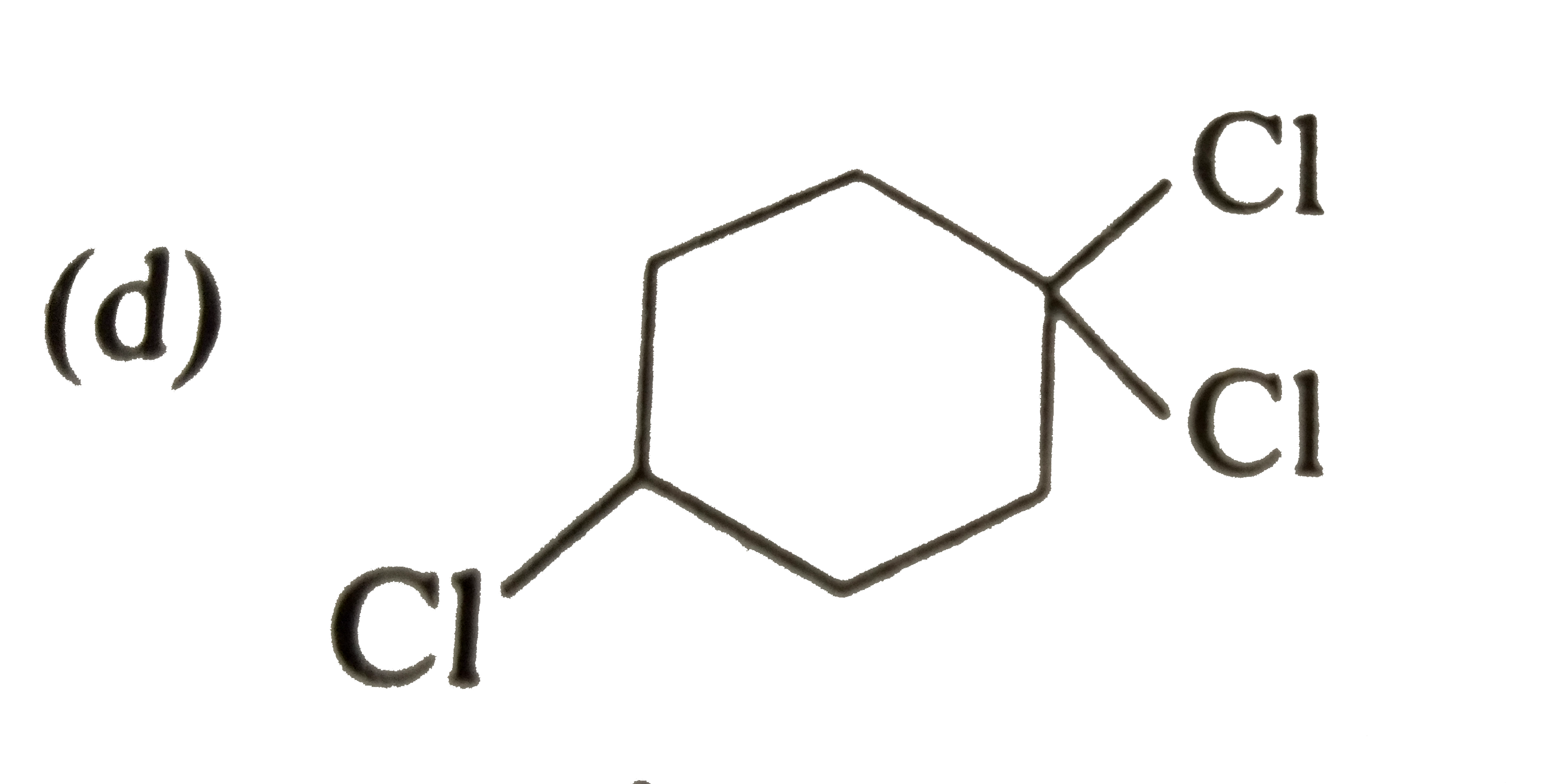
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
HIMANSHU PANDEY|Exercise Level 3 (Q.76 To Q.100)|25 VideosISOMERISM
HIMANSHU PANDEY|Exercise Level 3 (Q.101 To Q.125)|25 VideosISOMERISM
HIMANSHU PANDEY|Exercise Level 3 (Q.26 To Q.50)|25 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 VideosNOMENCLATURE
HIMANSHU PANDEY|Exercise Section Iv (Q.26 To Q.26)|1 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-ISOMERISM-Level 3 (Q.51 To Q.75)
- Compound X can exist in how many orientations ?
Text Solution
|
- Which of the following will not have any stereoismer ?
Text Solution
|
- Which of the following compounds of optically inactive
Text Solution
|
- Which of the following structures repersent meso compound
Text Solution
|
- How are the following compounds related
Text Solution
|
- Wich of the following paris of compounds is pair of enantiomers
Text Solution
|
- Which of the following compounds are meso froms
Text Solution
|
- How many isomers for the following molecule
Text Solution
|
- What are the correct designations for the structure below
Text Solution
|
- The following compounds differ in respect of
Text Solution
|
- How many structural isomers are possible when one of the hydrogen in ...
Text Solution
|
- Assign double bond configuration to the following
Text Solution
|
- Which of the following pairs are geometrical isomers
Text Solution
|
- The configuration of 1 and 2 carbon atom in the following compounds is
Text Solution
|
- are
Text Solution
|
- Which of the following conformers of 1,20diol cannot from intramolecul...
Text Solution
|
- Which of the following compounds does not have any geometrical isomer ...
Text Solution
|
- If a mixture of 2-bromobutane has enantiomeric excess of 50% of (+)-b...
Text Solution
|
- The following compounds differ in :
Text Solution
|
- How many stereomeres are possible for following molecule?
Text Solution
|