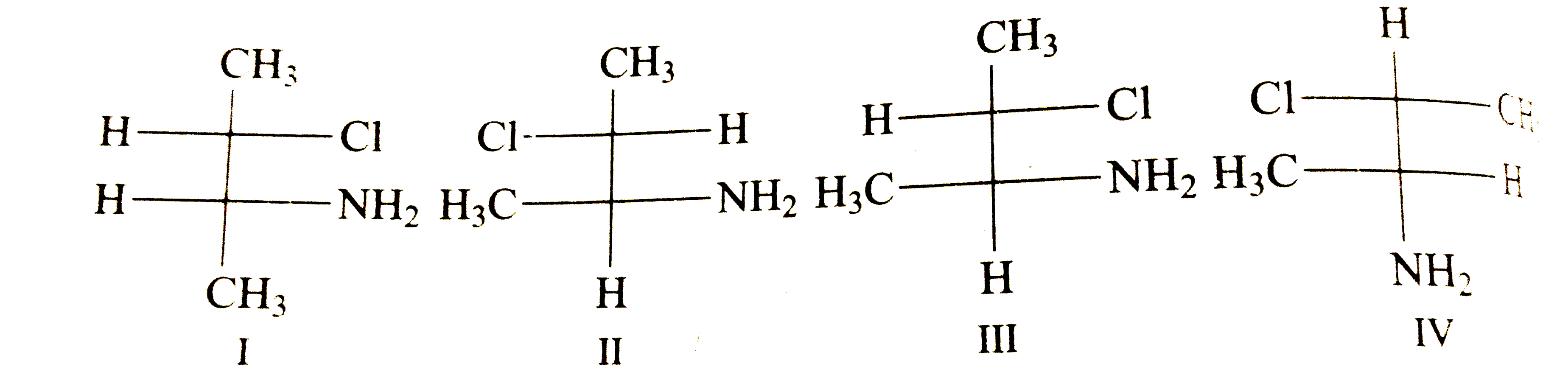A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
HIMANSHU PANDEY|Exercise Match The Column|5 VideosISOMERISM
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|12 VideosISOMERISM
HIMANSHU PANDEY|Exercise More Than One Correct (Q.26 To Q.45)|20 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 VideosNOMENCLATURE
HIMANSHU PANDEY|Exercise Section Iv (Q.26 To Q.26)|1 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-ISOMERISM-Linked Comprehension Type
- Presence of chiral center is not an essential condition to show optica...
Text Solution
|
- Presence of chiral center is not an essential condition to show optica...
Text Solution
|
- Presence of chiral center is not an essential condition to show optica...
Text Solution
|
- Cyclohexane exist as two chair conformations in rapid equilibrium at r...
Text Solution
|
- Cyclohexane exist as two chair conformations in rapid equilibrium at r...
Text Solution
|
- Cyclohexane exist as two chair conformations in rapid equilibrium at r...
Text Solution
|
- Conformations are different arragements of atoms htat are interconvert...
Text Solution
|
- Conformations are different arragements of atoms htat are interconvert...
Text Solution
|
- Conformations are different arragements of atoms htat are interconvert...
Text Solution
|
- A line which bisects a compound in two equal parts and both parts appe...
Text Solution
|
- A line which bisects a compound in two equal parts and both parts appe...
Text Solution
|
- A line which bisects a compound in two equal parts and both parts appe...
Text Solution
|
- R,S-configuration is a useful tool for determination of enantiomers, d...
Text Solution
|
- R,S-configuration is a useful tool for determination of enantiomers, d...
Text Solution
|
- R,S-configuration is a useful tool for determination of enantiomers, d...
Text Solution
|