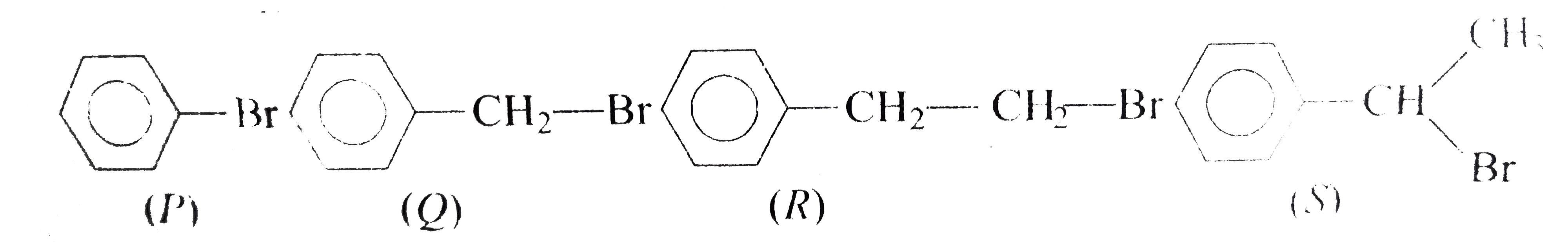
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HALIDES
HIMANSHU PANDEY|Exercise Level 2 (Q.76 To Q.100)|24 VideosHALIDES
HIMANSHU PANDEY|Exercise Level 2 (Q.101 To Q.125)|25 VideosHALIDES
HIMANSHU PANDEY|Exercise Level 2 (Q.26 To Q.50)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Subjective Type Problems|19 VideosISOMERISM
HIMANSHU PANDEY|Exercise Subjective Type Problems|11 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-HALIDES-Level 2 (Q.51 To Q.75)
- The correct decreasing order of relative reactivity of the following c...
Text Solution
|
- The relative reactivity of following halides toward S(N^(2)) reaction ...
Text Solution
|
- Rate of S(N^(1)) reaction is :
Text Solution
|
- In the following compound, arrange the reactivity of different bromine...
Text Solution
|
- Rate of reaction with aqueous ethanol follows the order :
Text Solution
|
- The reactivity of PhMgBr with the following compounds is :
Text Solution
|
- underset((P))(CH(3)Cl)" "underset((Q))(CH(3)CH(2)Cl)" "underset((R))(C...
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- H(3)C-O-CH(2)-Cl" "H(3)C-underset(O)underset(||)C-CH(2)-Cl" "H(3)C-CH(...
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|