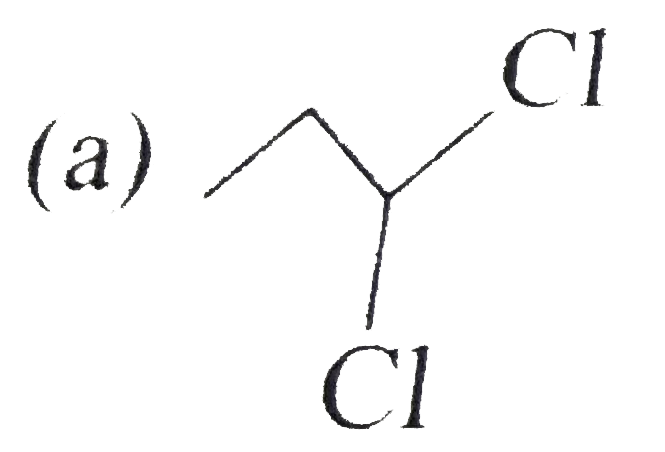
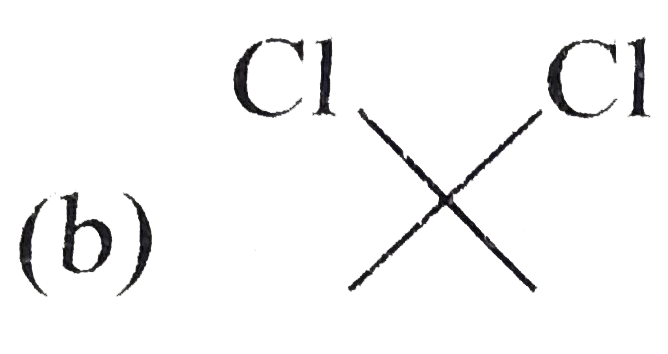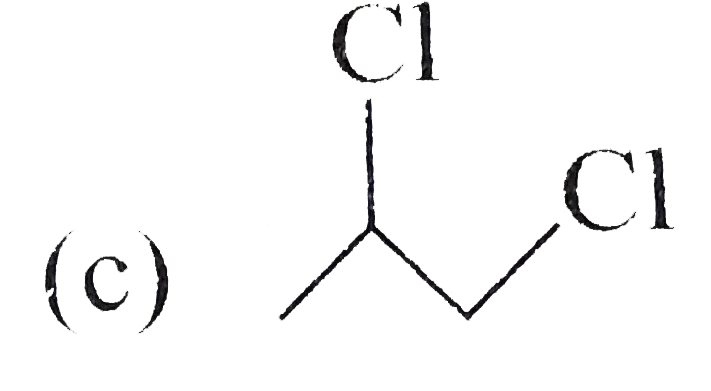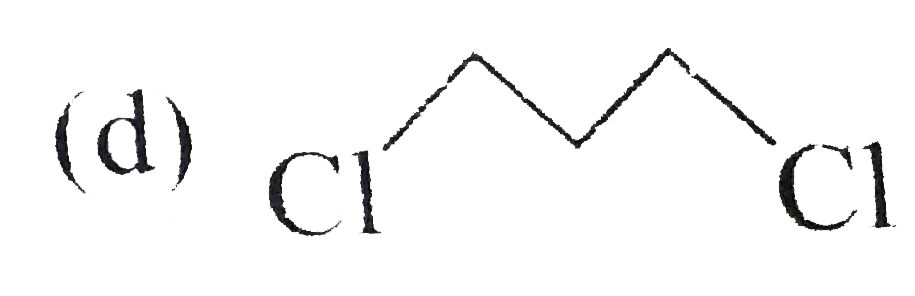A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALIDES
HIMANSHU PANDEY|Exercise Level 2 (Q.101 To Q.125)|25 VideosHALIDES
HIMANSHU PANDEY|Exercise Level 2 (Q.126 To Q.150)|25 VideosHALIDES
HIMANSHU PANDEY|Exercise Level 2 (Q.51 To Q.75)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Subjective Type Problems|19 VideosISOMERISM
HIMANSHU PANDEY|Exercise Subjective Type Problems|11 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-HALIDES-Level 2 (Q.76 To Q.100)
- Which is the correct order to stability?
Text Solution
|
Text Solution
|
- The reaction proceed by the mechanism :
Text Solution
|
- The reaction proceed by the mechanism :
Text Solution
|
- Consider the following reaction Which of the following products is ...
Text Solution
|
- The product formed in the reaction :
Text Solution
|
- Consider the following sequence of reactions underset(C(3)H(6)Cl(2))...
Text Solution
|
- Which of the following reactions will go faster if concentration of nu...
Text Solution
|
- Consider the following sequence of reaction Th product B is :
Text Solution
|
- Product Find the product :
Text Solution
|
- The order of decreasing nucleophilicities of the following species is ...
Text Solution
|
- The order of decreasing nucleophilicity of the following is :
Text Solution
|
- Consider the following nucleophiles When attached to sp^(3)-hybri...
Text Solution
|
- Find the product of the following reaction :
Text Solution
|
- Find the product of the following reaction :
Text Solution
|
- Find the major product of the following reaction :
Text Solution
|
- Find the major product of the following reaction :
Text Solution
|
- Find the major product of the following reaction :
Text Solution
|
- Which of the following reactions is not feasible ?
Text Solution
|
- Find the major product of the following reaction :
Text Solution
|