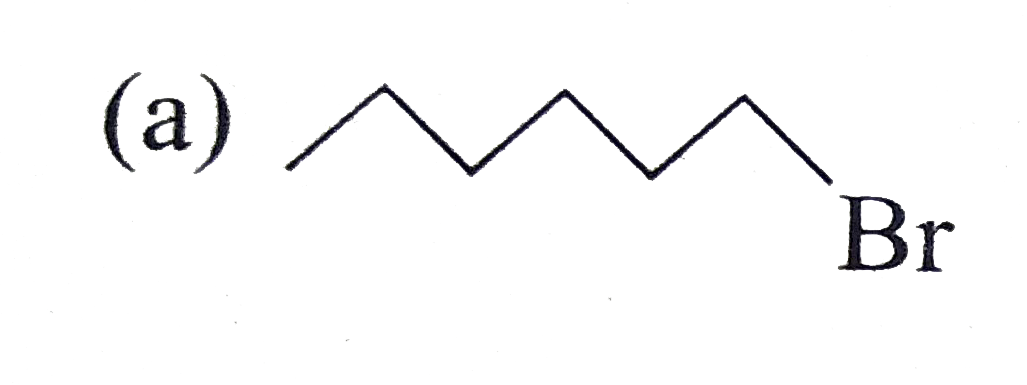
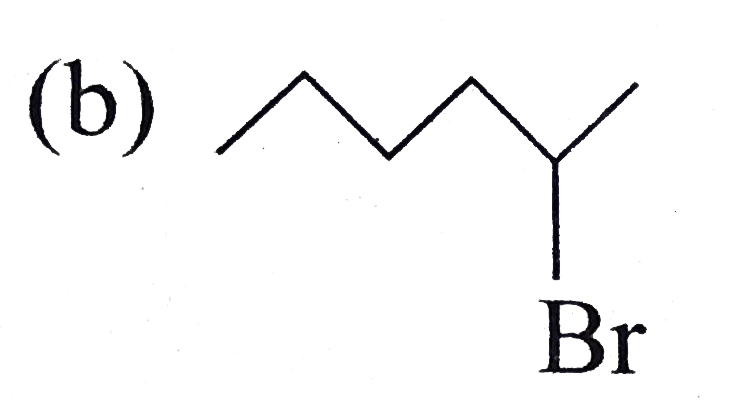
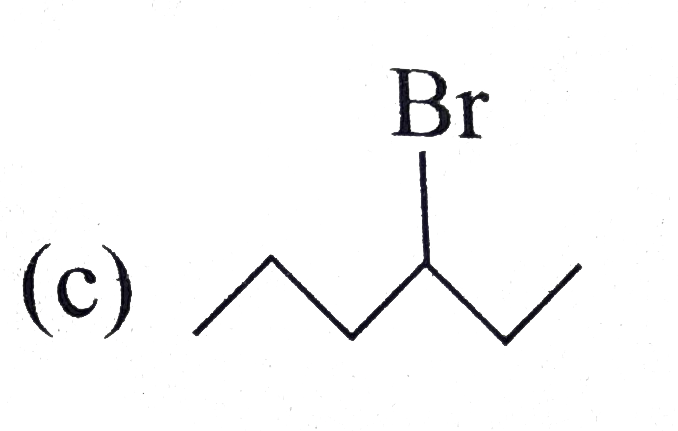
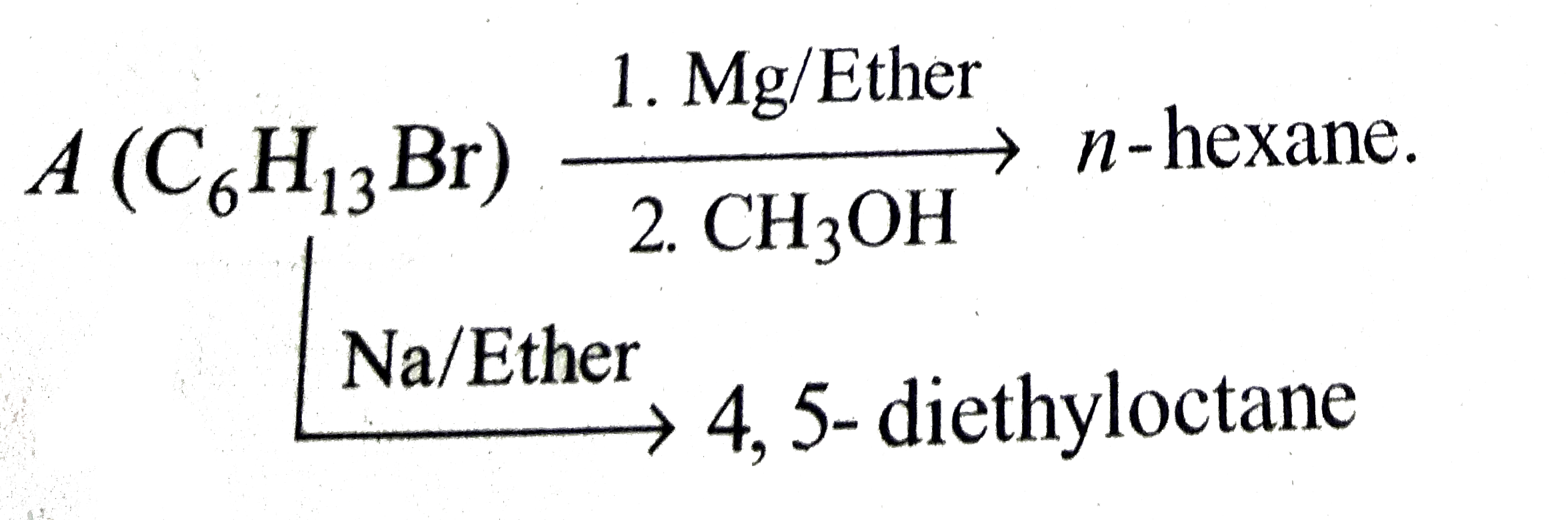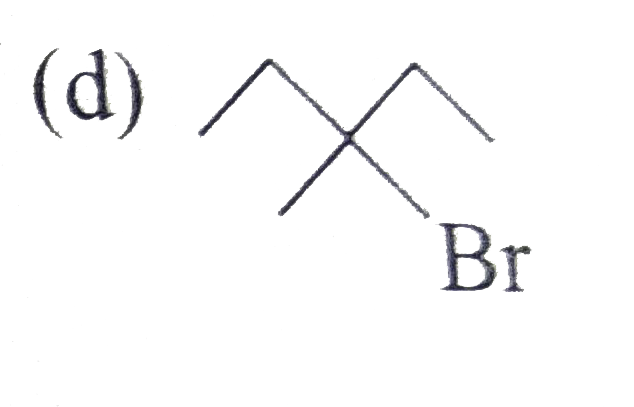A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HALIDES
HIMANSHU PANDEY|Exercise Level 2 (Q.151 To Q.165)|15 VideosHALIDES
HIMANSHU PANDEY|Exercise More Than One Correct (Q.1 To Q.25)|25 VideosHALIDES
HIMANSHU PANDEY|Exercise Level 2 (Q.101 To Q.125)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Subjective Type Problems|19 VideosISOMERISM
HIMANSHU PANDEY|Exercise Subjective Type Problems|11 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-HALIDES-Level 2 (Q.126 To Q.150)
- Complete the following reaction
Text Solution
|
- Major product :
Text Solution
|
- Ph-CH(2)-CH(2)overset(oplus)-overset(CH(2)CH(3))overset(|)underset(CH(...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Major product :
Text Solution
|
- Complete the following reaction
Text Solution
|
- Arrange the following in decreasing order of E(2) reaction :
Text Solution
|
- Which of the following is the correct option of regent for the given c...
Text Solution
|
- Which of the following is the correct option of reagent for the above ...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Deduce the structure of 'A' :
Text Solution
|
- Treatment of 2-bromobutane with hot alcoholic KOH gives a mixture of t...
Text Solution
|
- Find structure of 'C' :
Text Solution
|
- Major Product :
Text Solution
|
- Find the nature of folllowing reaction :
Text Solution
|
- Which of the following is the strucuture of A?
Text Solution
|
- Consider the following reaction Major product :
Text Solution
|
- Which reaction take place at the fastest rate?
Text Solution
|
- What are reactant X and product Y in the following sequence of reactio...
Text Solution
|
- What is the major product of following reaction?
Text Solution
|