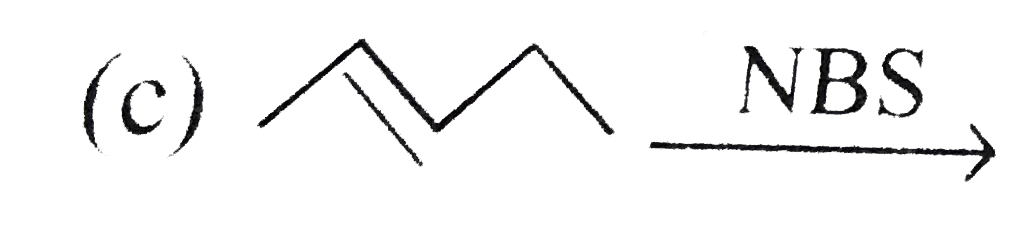
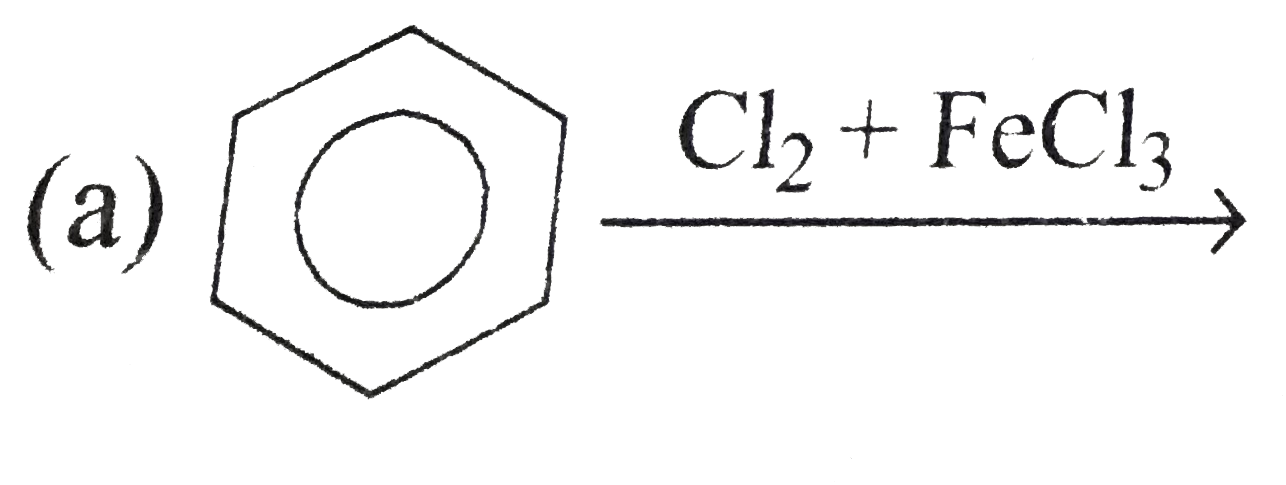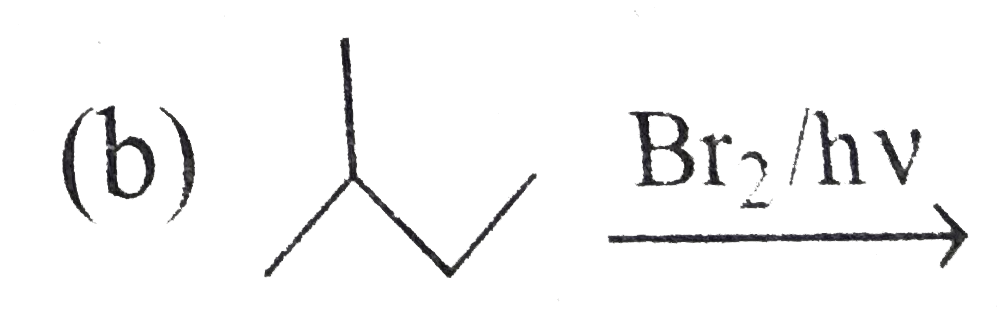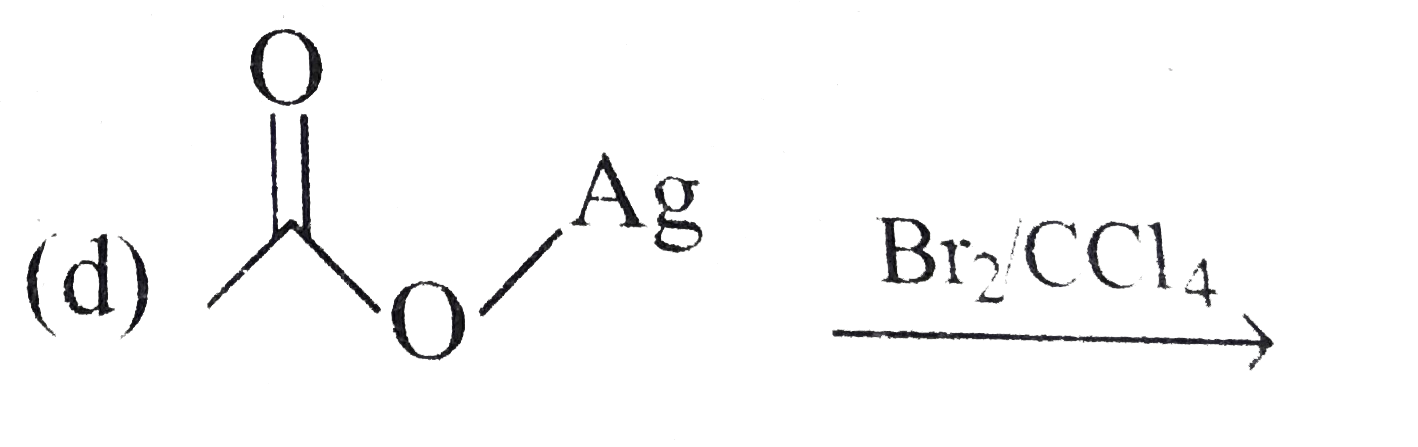A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALIDES
HIMANSHU PANDEY|Exercise Linked Comprehension Type (Q.1 To Q.25)|25 VideosHALIDES
HIMANSHU PANDEY|Exercise Linked Comprehension Type (Q.26 To Q.27)|2 VideosHALIDES
HIMANSHU PANDEY|Exercise More Than One Correct (Q.1 To Q.25)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Subjective Type Problems|19 VideosISOMERISM
HIMANSHU PANDEY|Exercise Subjective Type Problems|11 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-HALIDES-More Than One Correct (Q.26 To Q.50)
- Aryl halide undergo :
Text Solution
|
- Which of the following reactions take place by S(N^(2)) reaction?
Text Solution
|
- Which of the following reactions involve free radical as intermediate ...
Text Solution
|
- Which of the following are possible intermediate in the following reac...
Text Solution
|
- Which of the following compounds will give racemic mixture by S(N^(1))...
Text Solution
|
- Which of the following compounds will give E(1) reaction?
Text Solution
|
- X and Y :
Text Solution
|
- R-CH(2)-CH(2)-overset(Θ)Ooverset(oplus)Naoverset(CS(2))underset(CH(3)-...
Text Solution
|
- For the reaction Choose the correct statements :
Text Solution
|
- Which of the following can give E(1)cb reaction?
Text Solution
|
- Identify the compounds which may give NGP reaction :
Text Solution
|
- Which of the following reactions involve benzyne intermediate?
Text Solution
|
- Which of the following products can be obtained by S(N^(1)) reaction?
Text Solution
|
- Among the following pair of reactions in which pair second reaction is...
Text Solution
|
- Which of the following reactions produce the same product?
Text Solution
|
- Which of the following are correct order of nucleophilcity in CH(3)OH?
Text Solution
|
- Which of the following can give E(1)cb reaction in basic medium?
Text Solution
|
- Choose the correct comparison of reactivity toward E(2) reaction :
Text Solution
|
- Which of the following compounds cannot give E(2) reaction with strong...
Text Solution
|
- Which of the following are better leaving group than
Text Solution
|