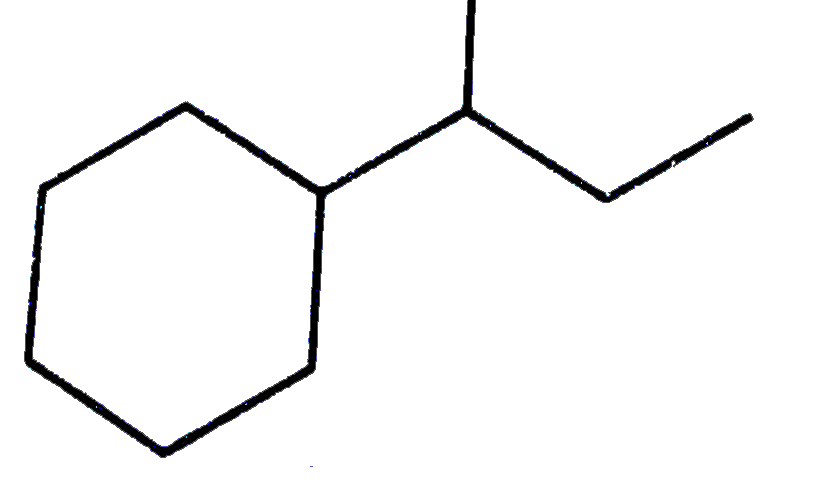
Text Solution
Verified by Experts
Topper's Solved these Questions
NOMENCLATURE
HIMANSHU PANDEY|Exercise Section Iv (Q.1 To Q.25)|25 VideosNOMENCLATURE
HIMANSHU PANDEY|Exercise Section Iv (Q.26 To Q.26)|1 VideosNOMENCLATURE
HIMANSHU PANDEY|Exercise Section Iii (Q.1 To Q.25)|25 VideosISOMERISM
HIMANSHU PANDEY|Exercise Subjective Type Problems|11 VideosPOLYMERS
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|8 Videos
Similar Questions
Explore conceptually related problems