A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NEET MOCK TEST 18-CHEMISTRY
- Which of the following has unpaired electron(s)?
Text Solution
|
- The pair of species having identical shapes for molecules of both spec...
Text Solution
|
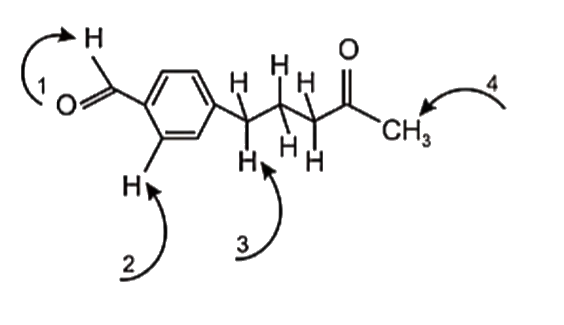
- Choose from the indicated protons, the one that is most acidic
Text Solution
|
- The products of the following chemical reactions are (i) C(2)H(5)NH(...
Text Solution
|
- Extraction of gold and silver involves leaching with CN^(-)ion.silver ...
Text Solution
|
- For the reaction mechanism of the reaction {:(2NO(g),+,2H(2)(g)),(to N...
Text Solution
|
- The reaction A(g) to B(g) + 2C(g) is a first-order reaction with a rat...
Text Solution
|
- Which of the following is an oxide ore ?
Text Solution
|
- CH3MgBr + CO(2)overset("Dry ether")(rarr)Y overset(H3O^(o+))(rarr)Z ...
Text Solution
|
- 3-Pentanol on reaction with aluminium tertiary butoxide in the presenc...
Text Solution
|
- In fluorite structure (CaF2)-
Text Solution
|
- 30 mL of 0.1MBaCl(2) is mixed with 40 mL of 0.2 M Al(2)(SO(4))(3). Wha...
Text Solution
|
- Identify the correct trend given below: (Atomic No = Ti : 22 , Cr : ...
Text Solution
|
- Sewage containing organic waste should not be disposed in water bodies...
Text Solution
|
- Densities of diamond and graphite are 3.5 and 2.3 g mL^(-1), respectiv...
Text Solution
|
- Which of the following will reduce Tollen's reagent ? Explain.
Text Solution
|
- Which of the following reaction(s) can be used for the preparation of ...
Text Solution
|
- Osmotic pressure of 40% (wt.//vol.) urea solution is 1.64 atm and that...
Text Solution
|
- Pb^(2+), Cu^(2+), Zn^(2+) and Ni^(2+) ions are present in a given acid...
Text Solution
|
- If two molecules of A and B having mass 100 amu and 64 amu respectivel...
Text Solution
|