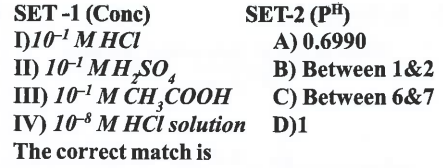
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NARAYNA-IONIC EQUILIBRIUM-Level-III
- In the equilibrium A^(-)+H(2)OhArrHA+OH^(-)(K(a)=1.0xx10^(-5)). The de...
Text Solution
|
- The pK(a) of HCN is 9.30. The pH of a solutin prepared by mixing 2.5 m...
Text Solution
|
- The K(b) for AgCl is 2.8 xx 10^(-10) at a given temperature. The solub...
Text Solution
|
- The molar solubility of AgCl in 1.8 M AgNO(3) solution is (K(sp) of Ag...
Text Solution
|
- 0.1 mole of CH(3)CH (K(b) = 5 xx 10^(-4)) is mixed with 0.08 mole of H...
Text Solution
|
- K(sp) of a salt ZnCl(2) is 3.2 xx 10^(-8) its P^(H) is
Text Solution
|
- In a saturated solution of the spatingly soluble strong electrolyte Ag...
Text Solution
|
- pH of saturated solution of Ba(OH)(2) is 12. The value of solubility p...
Text Solution
|
- The wight of HCl present in one litre of solution if pH of the soluti...
Text Solution
|
- A sample of AgCI was treated with 5.00mL of 1.5M Na(2)CO(3) solubility...
Text Solution
|
- The sulphide ion concentration [S^(2-)] in saturated H(2)S solution is...
Text Solution
|
- What is the pH of 0.01 M glycine solution? For glycine, K(a(1))=4.5xx1...
Text Solution
|
- The solubility of Mg (OH)(2) in pure water is 9.57 xx 10^(-3) gL^(-1)....
Text Solution
|
- The ionisation constant of an acid base indicator (a weak acid) is 1.0...
Text Solution
|
- An aqueous solution of a metal bromide MBr(2)(0.05M) is saturated with...
Text Solution
|
- A solution is saturated with respect to SrCO(3) and SrF(2). The [CO(3)...
Text Solution
|
- The maximum pH of a solution which is 0.10 M is Mg^(2+) from which Mg(...
Text Solution
|
- Match the following
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|