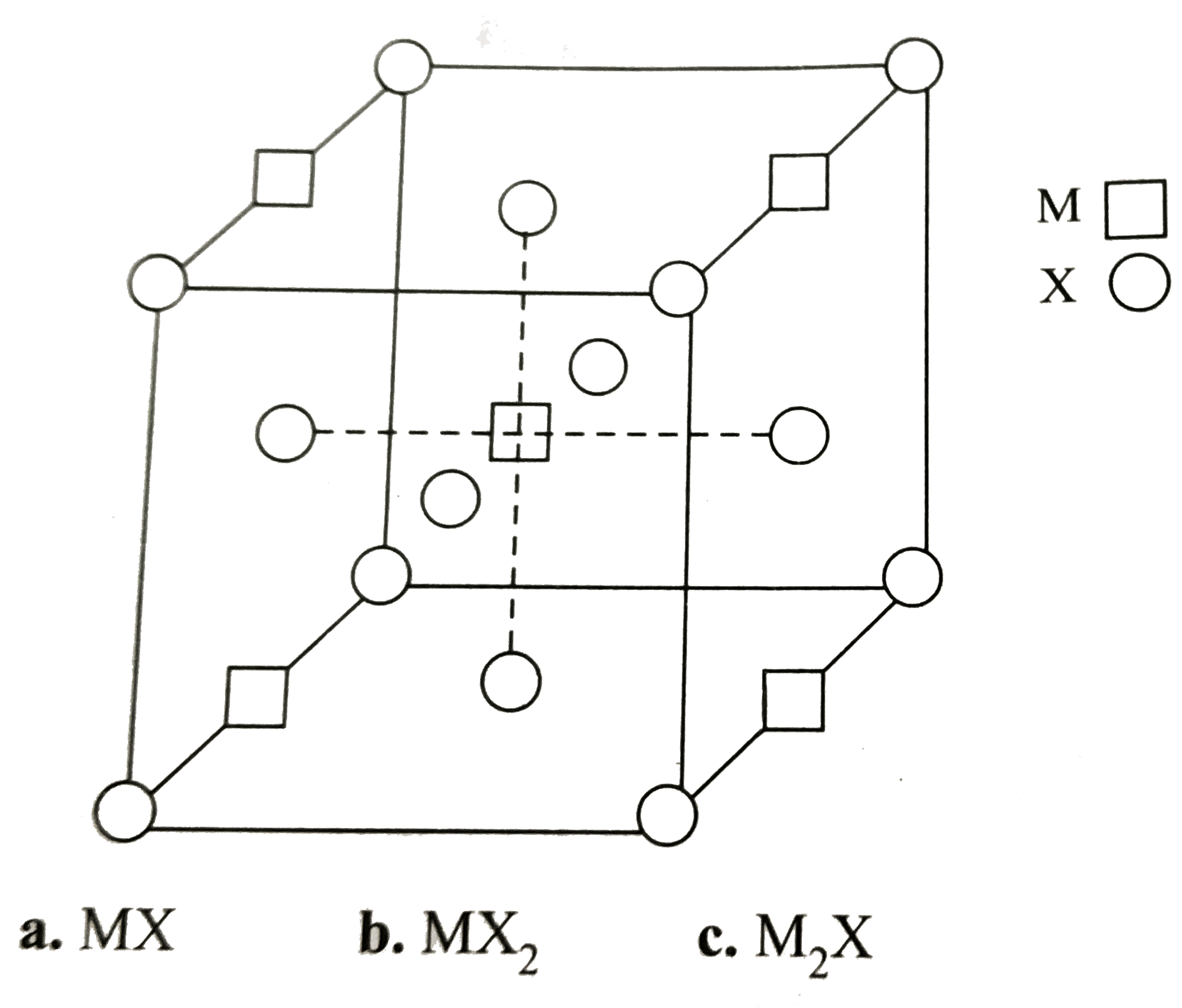
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-SOLID STATE-LEVEL-II(C.W)
- If the radius of K^(+) and F^(-) are 133pm and 136pm respectively, the...
Text Solution
|
- Number of unit cells in 4g of X(atomic mass=40). Which crystallises in...
Text Solution
|
- A metallic element has a cubic lattice. Each edge of the unif cell is ...
Text Solution
|
- In a hexagonal close packed (hcp) structure of spheres, the fraction o...
Text Solution
|
- In a structure, A atoms have fcc arrangement B atoms occupy all the te...
Text Solution
|
- In hexagonal closest-packed arrangement each spher has a coordinationn...
Text Solution
|
- In a close packed lattics containing 'n' particles, the number of tetr...
Text Solution
|
- A body centered cubic solid is made up of two elements A and B. Atoms ...
Text Solution
|
- A solid has a structure in which W atoms are located at the corners of...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- A metallic element crystallises into a lattice having a ABC ABC …… pat...
Text Solution
|
- If 'a' stands for the edge length of the cubic systems: simple cubic,b...
Text Solution
|
- A compound contains two types of atoms X and Y. it crystallises in a c...
Text Solution
|
- The volume of atom present in a face-centred cubic unit cell of a meta...
Text Solution
|
- The fraction of total volume occupied by atoms in a simple cube is
Text Solution
|
- a metal crystallizes with a face-centered cubic lattice.The edge of th...
Text Solution
|
- A semiconductor of Ge can be made p-type by adding
Text Solution
|
- Which one of the following ratio gives the purity of the metal (rho-re...
Text Solution
|
- Which of the following is correct statement
Text Solution
|
- In X-ray diffraction experiment at which one of the following path dif...
Text Solution
|