A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-SOLID STATE-LEVEL-III
- A solid AB has CsCl-type structure. The edge length of the unit cell i...
Text Solution
|
- Which is not correct about valence bond theory of metals
Text Solution
|
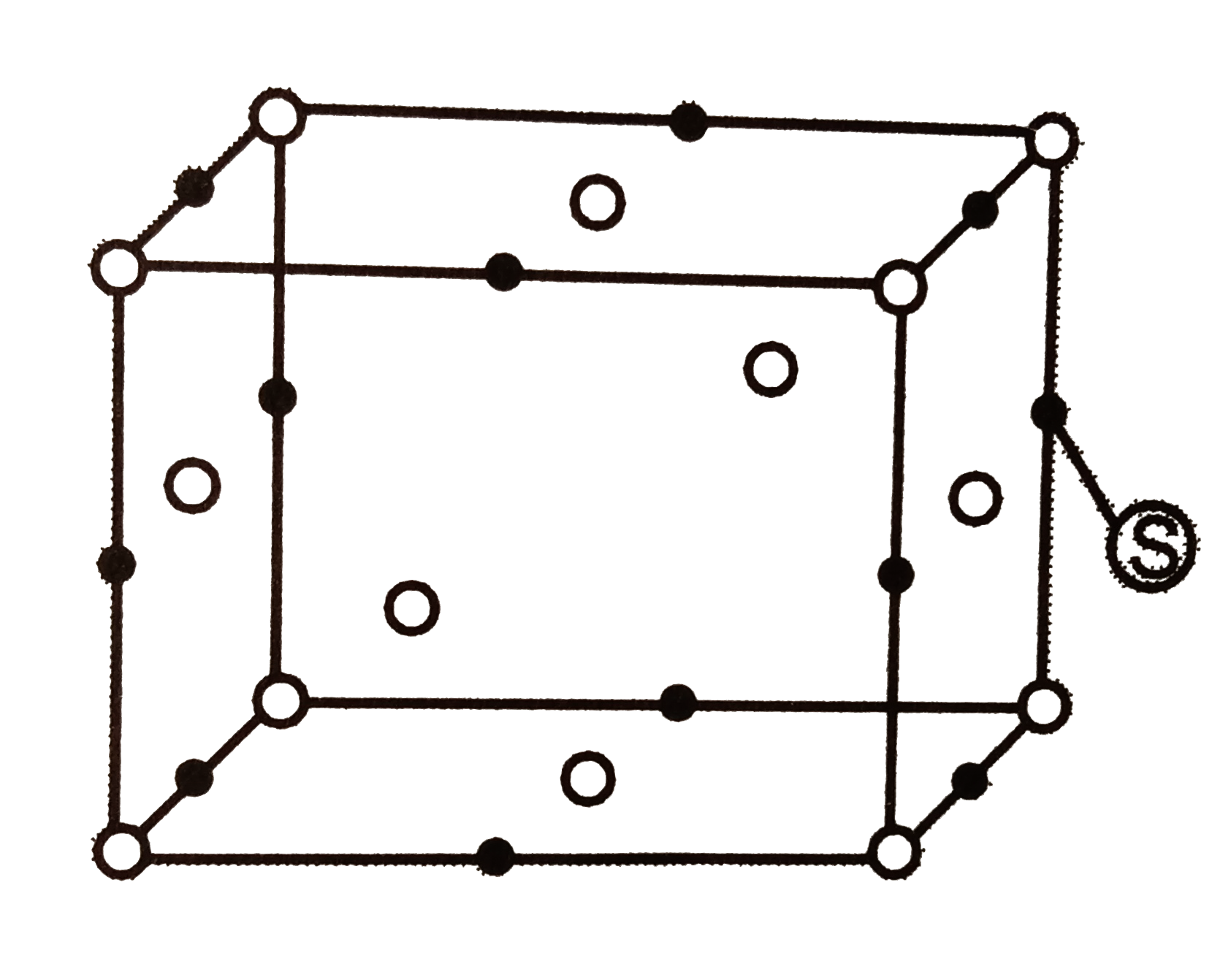
- For the structure given below, the site marked as S is a :
Text Solution
|
- Silica (SiO(2)) can be crystalline as well as amorphes with following ...
Text Solution
|
- If NaCl is doped with 10^(-3) mol percent of SrCI(2), what is the conc...
Text Solution
|
- Lithium selenide can be described as a closest- packed array of selen...
Text Solution
|
- Select right expression for determinig packing fraction (P.F.) of Na...
Text Solution
|
- whis is true
Text Solution
|
- Edge length of a cubic unit cell is 400pm. Its face diagonal would be
Text Solution
|
- Body diagonal of a cube is 866 pm. Its edge length would be
Text Solution
|
- The radius of Na^(+) is 95pm and that of Cl^(-) is 181 pm. The edge le...
Text Solution
|
- A metal crystallizes into two cubic phases, face-centred cubic and bod...
Text Solution
|
- An element cyrstallises in a 'bcc' lattice. Nearst niehgbours and perc...
Text Solution
|
- In a face centred cubic arrangement of A and B atoms whose A atoms are...
Text Solution
|
- Ice crystallises in hexagonal lattice having volume of unit cell is 13...
Text Solution
|
- Edge length of M^(+)X^(-) (fcc structure) is 7.2^(@)A. Assuming M^(+)-...
Text Solution
|
- A binary solid (A^(+)B^(-)) has a ruck salt structure. If the edge len...
Text Solution
|
- A solid has a bcc structure. If the distance of closest approach betwe...
Text Solution
|
- A metal crystallises as body centred cubic lattice with the edge lengt...
Text Solution
|
- The composition of a sample of wrustite is Fe(0.93)O(1.00).Percentage ...
Text Solution
|