A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-SOLID STATE-LEVEL-V
- A compound formed by elements X and Y has a cubic structure in whch X ...
Text Solution
|
- A compound formed by elements X and Y has a cubic structure in whch X ...
Text Solution
|
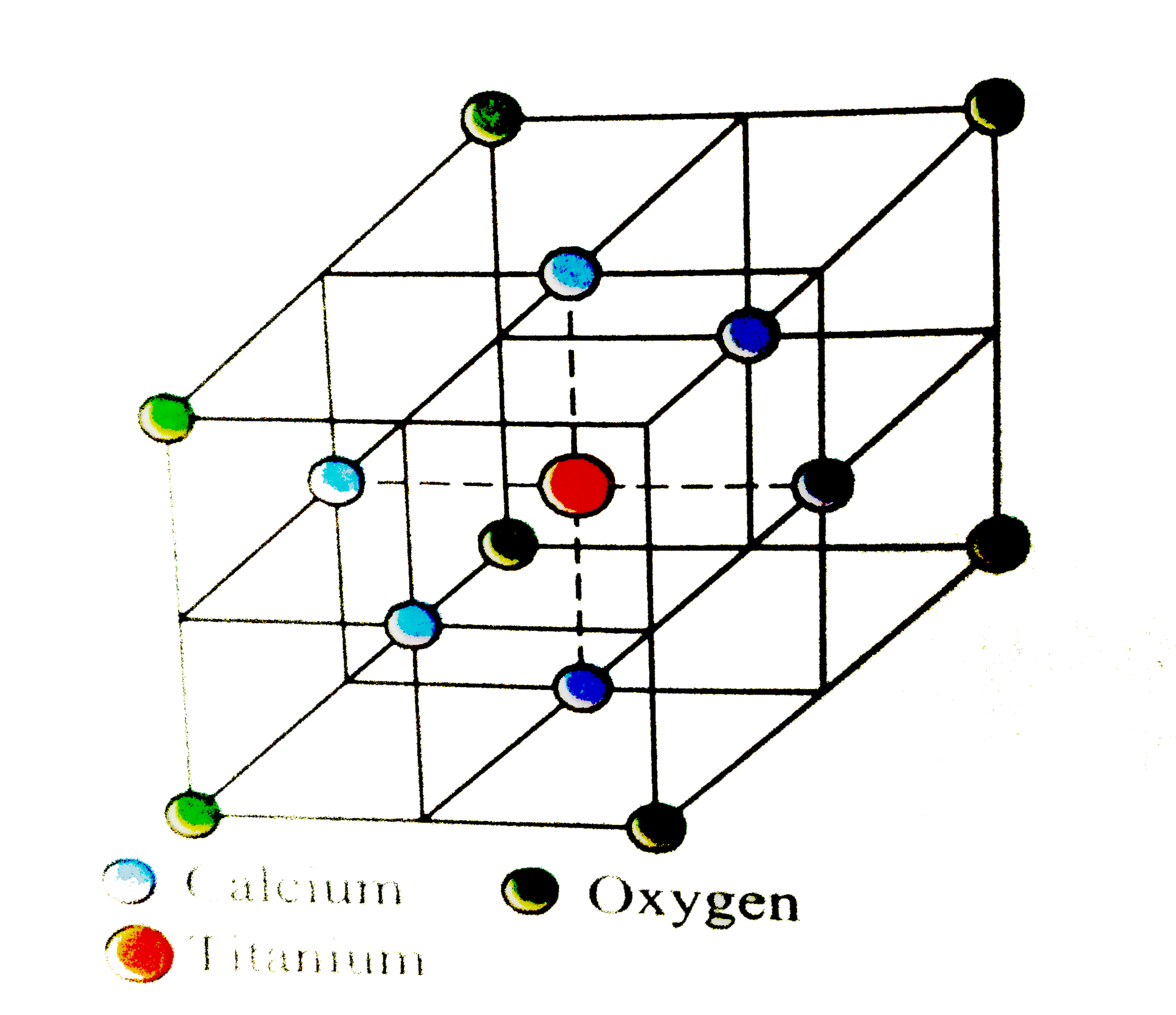
- Perovaskite, a mineral containing calcium, oxygen & titanium crystall...
Text Solution
|
- Perovaskite, a mineral containing calcium, oxygen & titanium crystall...
Text Solution
|
- Perovaskite, a mineral containing calcium, oxygen & titanium crystall...
Text Solution
|
- Ionic lattic has two major points defects ,(1) Schottky (2) Frenkel...
Text Solution
|
- Struture shown here repersents
Text Solution
|
- Struture shown here repersents
Text Solution
|
- Titanium (Ti) crystallizes in fcc lattice. It reacts with C or H inter...
Text Solution
|
- Titanium (Ti) crystallizes in fcc lattice. It reacts with C or H inter...
Text Solution
|
- Titanium (Ti) crystallizes in fcc lattice. It reacts with C or H inter...
Text Solution
|
- In zinc blende structure anions are arranged in ccp and cations are pr...
Text Solution
|
- In zinc blende structure anions are arranged in ccp and cations are pr...
Text Solution
|
- In zinc blende structure anions are arranged in ccp and cations are pr...
Text Solution
|
- An element occurs in the body centred cubic alttic with cell edge of 3...
Text Solution
|
- An element occurs in the body centred cubic alttic with cell edge of 3...
Text Solution
|
- An element occurs in the body centred cubic alttic with cell edge of 3...
Text Solution
|
- A solid made up of ions of A and B possess edge length of unit cell of...
Text Solution
|
- A solid made up of ions of A and B possess edge length of unit cell of...
Text Solution
|
- A solid made up of ions of A and B possess edge length of unit cell of...
Text Solution
|