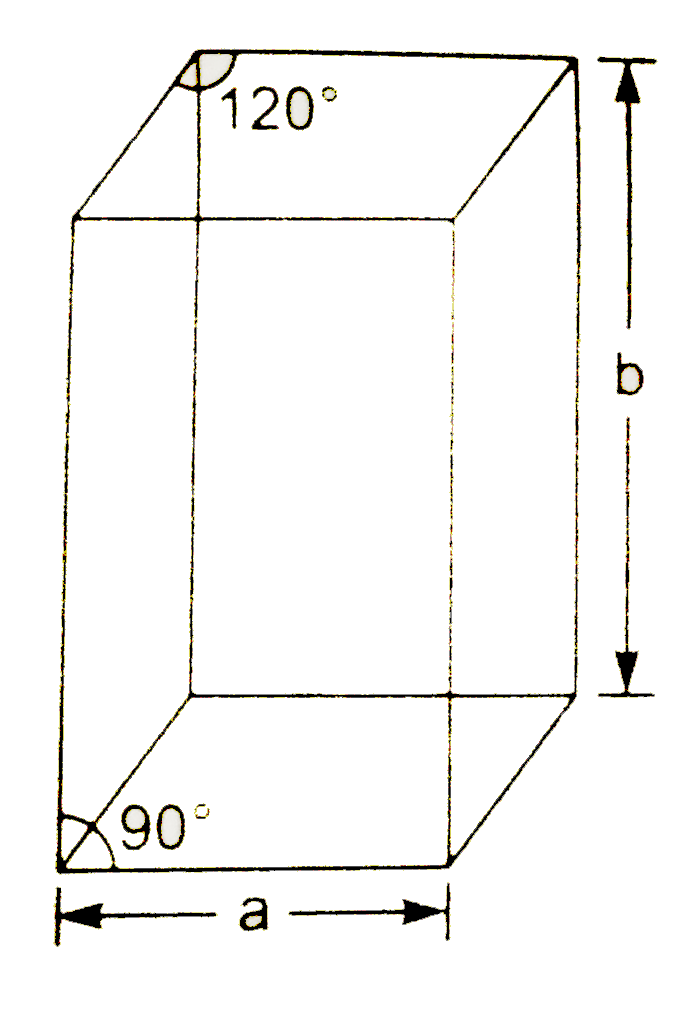
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A(2)B molecules(" molar mass " = 259.8 g//"ml") crystallises in a hex...
Text Solution
|
- A molecule A(2)B (Mw = 166.4) occupies triclinic lattice with a = 5 Å,...
Text Solution
|
- Ice crystallizes in a hexagonal lattice. At the low temperature at whi...
Text Solution
|
- A(2)B molecules (" molar mass " = 259.8 g//"ml") crystallises in a hex...
Text Solution
|
- Ice crystallizes in a hexagonal lattice. At ascertain low temperature,...
Text Solution
|
- Ice crystallizes in a hexagonal lattice. At the low temperature at whi...
Text Solution
|
- X=5Å Y=8Å Molar mass of solid ="259.8 g mol"^(-1) A solid crysta...
Text Solution
|
- बर्फ षट्कोणीय जालक में क्रिस्टलित होता है। निम्न ताप, जिस पर संरचना ज्...
Text Solution
|
- A molecule A(2)B (Mw = 166.4) occupies triclinic lattice with a = 5 Å,...
Text Solution
|