A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
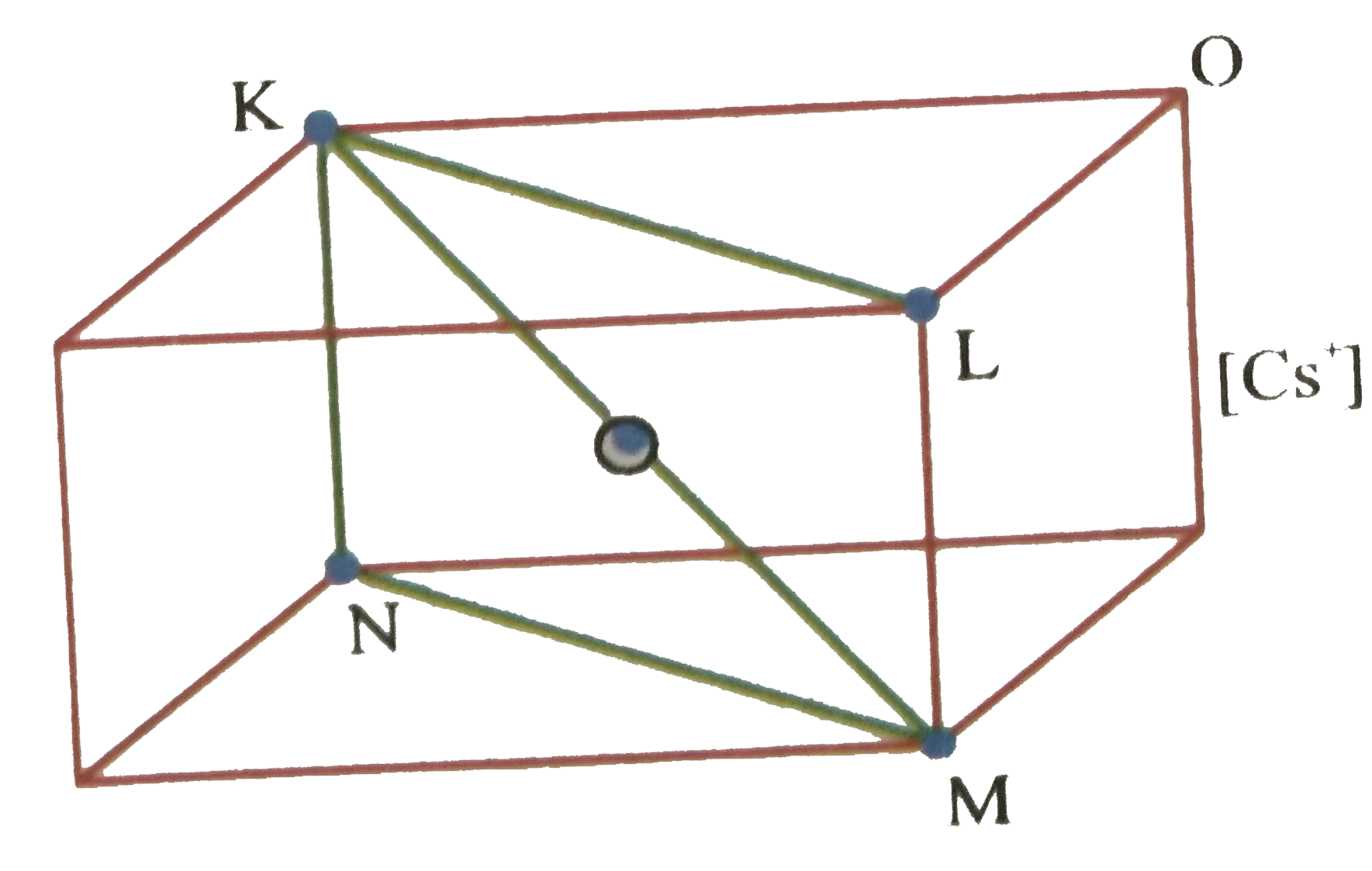
- CsCl structure is given below , Assign all contact with cation and ani...
Text Solution
|
- CsCl has bcc structure with Cs^(+) at the centre and Cl^(-) ion at eac...
Text Solution
|
- A salt AB cystallises in the CsCl structure. The anions at the corners...
Text Solution
|
- CsCl structure is given below , Assign all contact with cation and ani...
Text Solution
|
- The edge length of LiCl (NaCl structure) unit cell is 514 pm. Assumi...
Text Solution
|
- Match List I with List II and select the correct answer using the code...
Text Solution
|
- Percentage of void space in AB solid having rock salt structure if (r(...
Text Solution
|
- Assertion. If the length of the unit cell of LiCl having NaCl structur...
Text Solution
|
- Assertion.If the length of the unit cell of LiCl having NaCl structure...
Text Solution
|