A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CABOXYLIC ACIDS AND ITS DERIVATIVES
HIMANSHU PANDEY|Exercise Level 1 (Q.27 To Q.50)|24 VideosCABOXYLIC ACIDS AND ITS DERIVATIVES
HIMANSHU PANDEY|Exercise Level 2 (Q.1 To Q.25)|25 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise Match The Column|6 VideosCARBONYL COMPOUNDS
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-CABOXYLIC ACIDS AND ITS DERIVATIVES-Subjective Type Problems
- on saponification forms:
Text Solution
|
- Find out products of following reactions.
Text Solution
|
Text Solution
|
Text Solution
|
Text Solution
|
Text Solution
|
- Ph-CH(2)-Cloverset(Mg)underset(Ether)rarroverset(CO(2))underset(H^(opl...
Text Solution
|
Text Solution
|
- PhMgBroverset(CO(2))underset(H^(oplus)//HOH)rarroverset(SOCl(2))rarrov...
Text Solution
|
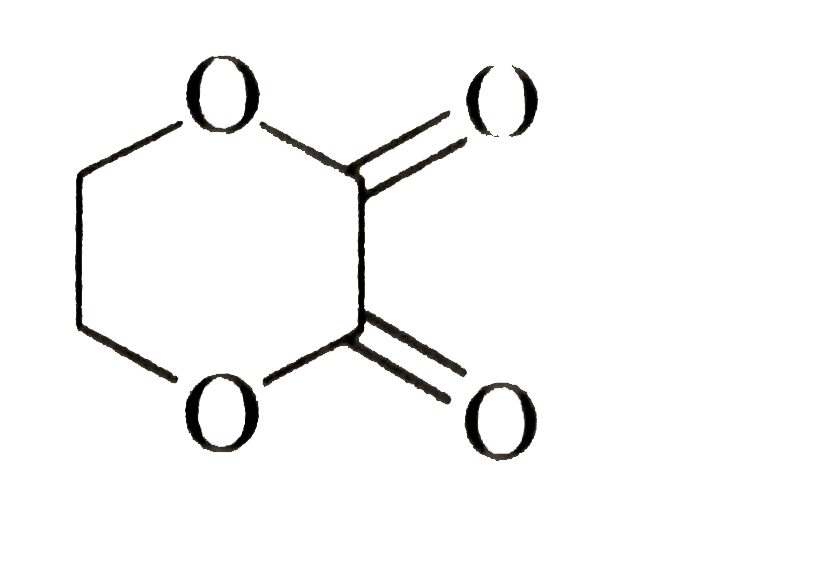 on saponification forms:
on saponification forms: